Relationships between Nocturia, Obstructive Sleep Apnea, and Quality of Sleep
Article information
Abstract
Background and Objective
Nocturia is one of the common causes of sleep disturbance in adults, often associated with obstructive sleep apnea (OSA). The purpose of this study was to investigate the association between nocturia and OSA, and their effects on quality of sleep in adults.
Methods
This study included 107 patients who visited the urology outpatient due to lower urinary tract symptoms with or without sleep-related symptoms, assessed by using either the International Prostate Symptom Score in men or the Overactive Bladder symptoms in women. Based on these questionnaires, subjects were divided into nocturia and nocturia-free groups. In addition, all subjects completed detailed sleep questionnaires including the Global Sleep Assessment Questionnaire (GSAQ) and the Berlin Questionnaire (BQ), and provided details of voiding volumes and times. Night polysomnography (PSG) was performed in subjects with high risk of OSA, as indicated by the BQ.
Results
Among the 107 study subjects (43 males, 64 females, mean age 59.2 ± 11.2 years), nocturia was present in 54.2% (58/107), self-reported sleep disturbance in 26.2% (28/107), and a high risk of OSA, with BQ > 10 points, was present in 29.0% (31/107) of subjects. The nocturia group had a significantly higher mean GSAQ total score than the nocturia-free group, indicating poorer sleep quality (p = 0.004). Nocturia was more frequent in patients with moderate or severe OSA than in patients with mild OSA (p = 0.006) based on PSG.
Conclusions
Nocturia was a clinically significant sleep disturbing factor, and it was more frequent in patients with more severe OSA.
INTRODUCTION
Nocturia, the condition of waking up to urinate one or more times during the night, is common in adults, with a reported prevalence of 53%.1 However, the potential contribution that leads to sleep disturbance is less well acknowledged.2,3 Nocturia has been reported to be an independent predictor of self-reported insomnia with a 75% increased risk and of sleep quality reduction with a 71% increased risk.1 Many causes of frequent nocturnal awakening to void have been described in the adult population. These include decreased bladder capacity, reduced glomerular filtration rate, and nocturnal polyuria due to decreased arginine vasopressin, incipient diabetes, sleep-disordered breathing, such as, obstructive sleep apnea (OSA), congestive heart failure and/or diuretics use, benign prostatic hypertrophy in men and vaginal atrophy with consequent reduced sphincter control in women.4 Nocturia has been reported to be more severe in patients with OSA,5,6 and it is a well-researched end-organ symptom of OSA.7 In a previous study,8 OSA was found to be frequently associated with nocturia: 81% (17/21) women with OSA versus 40% (4/10) control women without OSA. The mechanism responsible for a higher prevalence of nocturia in OSA patients could be due to a reduced ability to concentrate urine during night sleep. OSA is a condition characterized by repetitive episodes of complete or partial upper airway obstruction, which causes negative intrathoracic pressure and consequently increases venous return to the heart.9–13 As a result, the right atrium and ventricle are distended, which receives wrong signaling of fluid overload, and the consequent release of brain-type atrial natriuretic peptide (ANP) from cardiac atria and ventricles.12,14,15 The secretion of antidiuretic hormone (ADH), which is increased at night to promote the absorption of sodium and water from collecting tubules, is subsequently inhibited and this eventually leads to decreased urine concentration and consequent polyuria during night sleep.15–18 In another study,8 diluted night-time urine was noted in 80% (16/21) of OSA patients, whereas it was noted in only 30% (3/10) of the control group. Numerous studies have reported reduction in nocturia after continuous positive airway pressure (CPAP) treatment in OSA patients,9–11,13,18,19 which supports the idea that OSA itself induces nocturia. However, despite the well-known pathophysiologic link between nocturia and OSA described above, the importance of nocturia has not been emphasized sufficiently in the context of screening for OSA.
The present study was performed to evaluate the prevalence of nocturia and its impact on sleep disturbance in adults who visited our urology outpatient clinic due to lower urinary tract symptoms including nocturia, and to investigate the impact of nocturia on sleep quality and the relationship between nocturia and OSA.
METHODS
Subjects were recruited from among patients who had visited our Urology outpatient clinic at Ewha Medical Center from September 2009 to September 2010 with lower urinary tract symptoms including nocturia (nocturnal frequency), nocturnal polyuria, urinary storage, urination, and post-micturition symptoms. All patients were asked to answer the International Prostate Symptom Score (IPSS) and Overactive Bladder (OAB) questionnaires about urination and urination-related symptoms within the previous 4 weeks.20–24 The IPSS and OAB questionnaires were used for men and women, respectively, to assess urination-related symptoms. Both questionnaires included the question, “How many times on average do you wake up to urinate during sleep?”. Patients were asked to record voiding times and corresponding voiding volumes for the recorded times on the three consecutive days, and to visit our urology outpatient clinic for uroflowmetry and post-void residual urine volume measurement. Nocturnal polyuria was defined to be present when the percentage night-time voided volume divided by total voided volume per day exceeded 33% using 3-day urinary frequency and volume charts. All subjects were screened for quality of sleep after obtaining their informed consent, and total 107 subjects completed the screening questionnaire. To evaluate the impact of nocturia on sleep quality and the relationship between nocturia and OSA, we allocated these subjects to the nocturia or nocturia-free (control) groups. We allocated the subjects who reported at least one episode of awakening during sleep for voiding over the past 4 weeks to the nocturia group.21–23
The Medical Outcomes Survey Sleep Scale (MOS-S), Global Sleep Assessment Questionnaire (GSAQ), Insomnia Severity Index (ISI), Epworth Sleepiness Scale (ESS), and the Berlin Questionnaire (BQ) were used as sleep-related questionnaires, and the MOS-S and GSAQ were used to assess general sleep quality. The total GSAQ scores in the nocturia and control groups were also compared. To evaluate self-reported insomnia, responses to individual questions and total ISI scores were compared. Patients with total ESS scores ranging from 10 to 24 were considered to have meaningful daytime sleepiness. The risk of sleep apnea syndrome was assessed using the BQ in all study subjects. Subjects with a BQ score of 0 or 1 point to each question (total 9 points) were considered to belong to the low risk group, and subjects with a BQ score ≥ 2 points to each question (total 10 points) were considered to belong to the high risk group. Among these subjects who completed the sleep questionnaires, patients with high risk of sleep apnea who had a score higher than total 10 points indicated by the BQ were referred to the sleep laboratory for full-night nocturnal polysomnography (PSG). Severity of OSA was graded as mild, moderate, or severe, based on the apnea-hypopnea index (AHI) and the lowest SaO2 from PSG indices–mild (5 ≤ AHI < 15 or 85% < lowest SaO2 < 90%), moderate (15 ≤ AHI < 30 or 75% ≤ lowest SaO2 ≤ 85%), or severe (AHI ≥ 30 or lowest SaO2 < 75%). We then compared the mild, moderate, and severe OSA groups with respect to demographics (age and gender), prevalence of nocturia, total IPSS score, and several sleep parameters (GSAQ, ISI, ESS). In addition, these groups were compared in terms of sleep disturbing factors, such as, pain, worry, drugs, nocturia, and underlying disease.
Statistical analysis was performed using the student’s t-test for comparing all variables between subjects with nocturia and without nocturia. The Kruskal-Wallis test was used to analyze the effects of demographics (age, sex), prevalence of nocturia, total IPSS score, and several sleep parameters (GSAQ, ISI, ESS) in the three OSA groups, and one-way analysis of variance was used to analyze the effects of sleep disturbing factors. All statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was accepted for p values < 0.05.
RESULTS
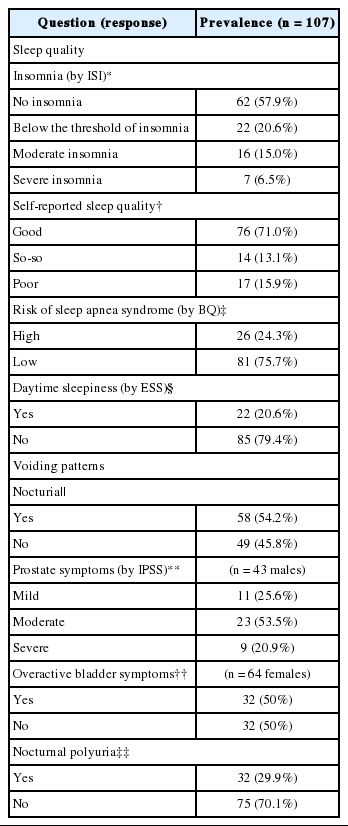
The total number of study subjects recruited in this study was 107, of which 43 were men and 64 were women. Mean age was 59.2 ± 11.2 years. Of these, 29.0% (31/107) reported sleep disturbance. Insomnia of moderate to severe degree was reported by 21.5% of subjects and daytime sleepiness by 20.6% of subjects. The BQ indicated a high risk of OSA in 24.3% of the patients (Table 1). Nocturia was found to be one of the main factors, with the highest proportion of patients replying that the factor disturbed sleep “often or always”. Of the 107 study subjects, 54.2% suffered from nocturia, 74.4% from moderate to severe prostate symptoms, 50% had OAB symptoms, and 29.9% had nocturnal polyuria (Table 1).
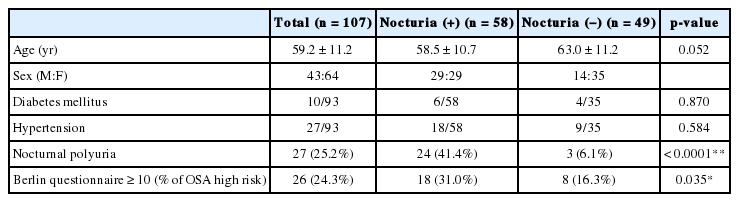
When dichotomized based on the presence of nocturia, 58 patients were allocated to the nocturia group and 49 patients were allocated to the control group. No significant inter-group difference was found in mean age, prevalence of diabetes mellitus, or hypertension (Table 2). On reviewing MOS-S, subjects in the nocturia group took more time to fall asleep (2.39 ± 1.46 hours in the nocturia group versus 1.77 ± 1.16 hours in the control group). The prevalence of awakening short of breath or with a headache, daytime sleepiness, or difficulty in falling asleep initially or again after awakening was significantly greater in the nocturia group with p-values of 0.028, 0.042, 0.004, and 0.017, respectively (Table 3). On analyzing individual questions in the GSAQ, subjects in the nocturia group were found to have more frequent difficulty staying asleep or felt poorly rested in the morning (p = 0.019), were more frequently prevented from getting enough sleep due to work or other activities (p = 0.016), to have ‘restless’ or ‘crawling’ feeling in the legs at night (p = 0.102), or to have repeated rhythmic leg jerks or leg twitches during sleep (p = 0.090) (Table 3). On reviewing the ISI questions, the subjects in the nocturia group more frequently complained of difficulty falling asleep than those in the control group (p = 0.001) (Table 4). The ESS showed no significant inter-group difference in terms of meaningful daytime sleepiness (Table 4). The prevalence of high risk for OSA by the BQ was significantly higher in the nocturia group (p = 0.035) (Table 2).
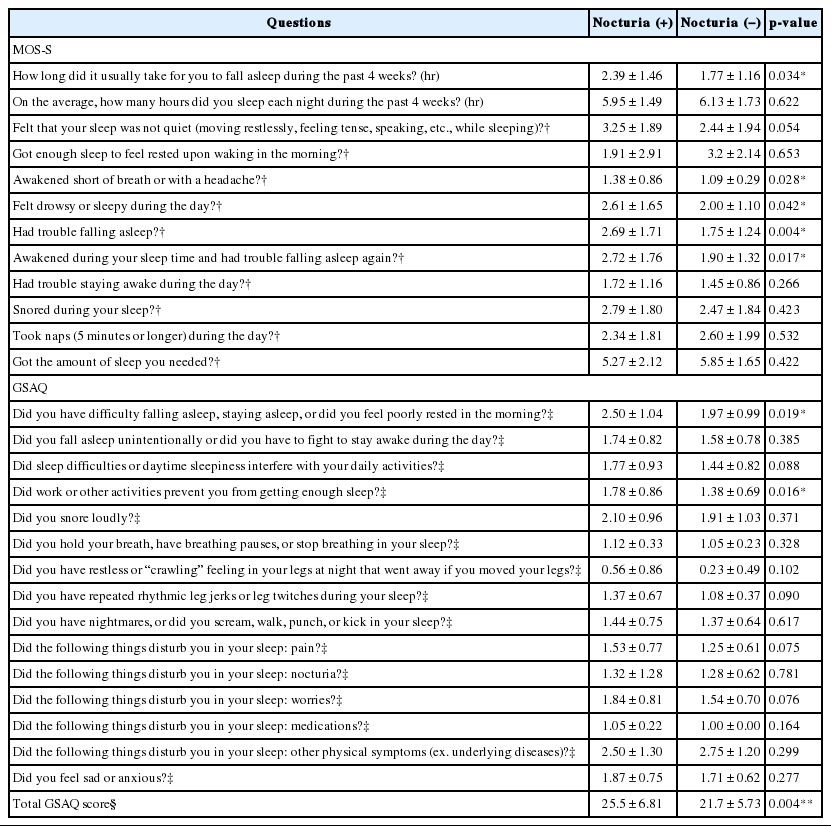
Medical Outcomes Survey Sleep Scale (MOS-S) and Global Sleep Assessment Questionnaire (GSAQ) scores in patients with and without nocturia
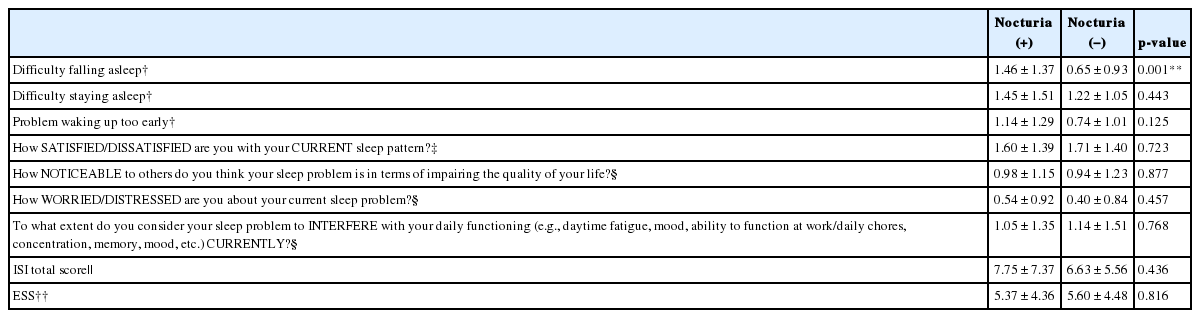
Insomnia Severity Index (ISI) and Epworth Sleepiness Scale (ESS) in patients with and without nocturia
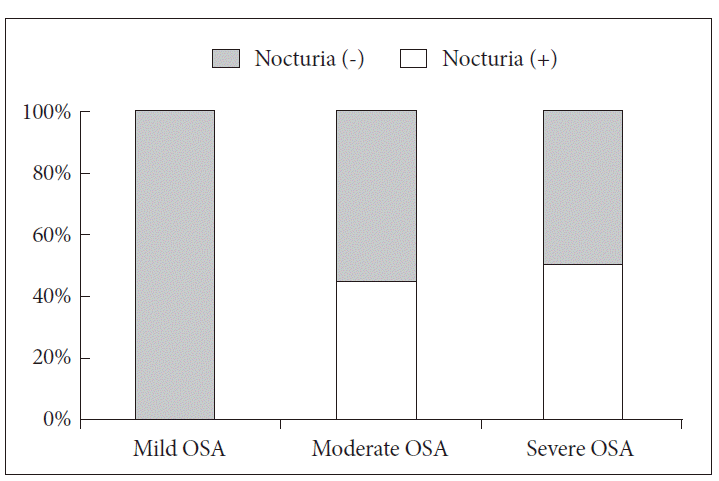
Night PSG was conducted in 26 patients with high risk for OSA based on the BQ, and all of them were diagnosed to have OSA of varying degrees; 7 had mild OSA (mean AHI of 3.98, mean lowest SaO2 89.5%), 9 had moderate OSA (mean AHI of 31.31, mean lowest SaO2 80.7%), and 10 had severe OSA (mean AHI of 48.20, mean lowest SaO2 73.5%). No significant differences were found between these three groups with respect to age, sex, IPSS score, GSAQ, ISI, or ESS (Table 5). Interestingly, the prevalence of nocturia was significantly higher in patients with moderate to severs OSA (p = 0.019 by Kruskal-Wallis test). More specifically, none of the patients with mild OSA reported nocturia (0%), whereas about half of the patients with moderate to severe OSA complained of nocturia (44.4% of patients with moderate OSA and 50.0% of patients with severe OSA) (Fig. 1). When individual sleep-disturbing factors were analyzed, the prevalence of nocturia was found to increase with OSA severity (p = 0.006) (Table 5).
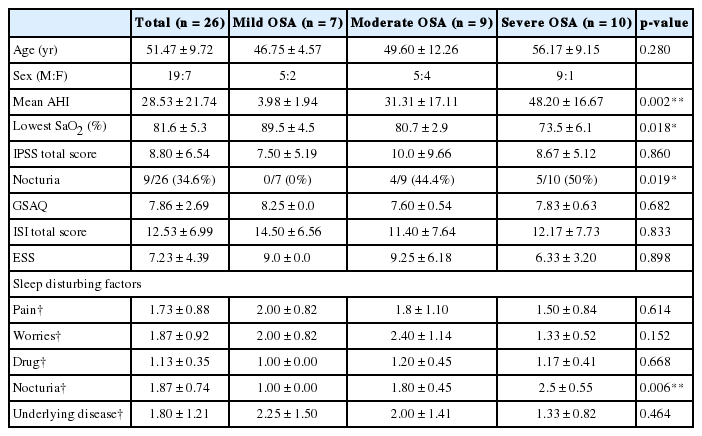
Demographics, urinary/sleep parameters, sleep disturbing factors, and the prevalence of nocturia according to OSA severity based on PSG findings
DISCUSSION
In the present study, we evaluated the relationship between nocturia and sleep problems in 107 adults older than 35 years who visited our urology clinic and who complained of any sleep-related symptoms. In these patients, nocturia was found to be present in 54.2% (58/107), which is consistent with the finding of a previous study that reported the presence of nocturia in 52.53% of 1424 adult patients with sleep-related symptoms aged between 55–84 years.1 Self-reported sleep disturbance in whatever form was noted in 29.0% of the study subjects. Among them, nocturia was suggested as a possible sleep-disturbing factor, especially preventing individuals from falling asleep, as indicated by the MOS-S, GSAQ, and ISI questionnaires. The MOS-S and GSAQ questionnaires also showed that the subjects in the nocturia group reported disturbed sleep and felt poorly rested in the morning more frequently. Furthermore, nocturia was found more often in patients with moderate to severe OSA compared to patients with mild OSA (p = 0.006).
Recently, nocturia has received more attention as a common end-organ symptom of OSA,7 and it has been emphasized that nocturia and snoring are predictive symptoms of OSA.25 In our study, nocturia was found to be closely related with symptoms suggestive of OSA, such as shortness of breath or a headache during sleep, based on the MOS-S questionnaire findings. Shortness of breath or headache during sleep could be the result of oxygen desaturation due to OSA, which implies that patients with nocturia have an increased likelihood of a co-existing medical condition causing oxygen de-saturation during sleep. In addition, the subjects in the nocturia group had a higher risk of OSA as indicated by the BQ (p = 0.035), which suggests that nocturia is significantly more prevalent in patients with OSA. Furthermore, nocturia was more commonly observed in patients with moderate to severe OSA than in patients with mild OSA, and nocturia was found to be more common in patients with more severe forms of OSA (nocturia was present in 0% of patients with mild OSA, in 44.4% of patients with moderate OSA, and in 50% of patients with severe OSA). This finding indicates a close relationship between OSA and nocturia.
It has been hypothesized that OSA induces increasingly negative intrathoracic pressure swings due to upper airway obstruction during sleep, which in turn increases the venous return,9–13 and that subsequently an enlarged right atrium receives a false alarm of fluid overload, which leads to increased ANP release from the right atrium and ventricle, and increased diuresis induced by ADH and aldosterone (responsible for regulating the hormonal system).12,14–17 Furthermore, the more severe is the OSA, the more likely it is that this vicious cycle aggravates, and worsens nocturia. In the light of this current knowledge,9–17 it might be expected that in OSA, nocturia occurs in the form of nocturnal polyuria due to urine concentration at night. In the present study, the presence of nocturnal polyuria was statistically more prevalent in the nocturia group than in the nocturia-free control group, and when it is considered that the subjects in the nocturia group were found to have a higher risk of OSA, it may be possible that nocturnal polyuria is directly related to OSA. Furthermore, the above-mentioned pathophysiologic mechanism of nocturia in OSA patients supports this possibility.
To provide stronger, objective evidence that supports more detailed mechanisms of nocturia in OSA, additional investigations are required, especially by measuring the hormone levels, such as those of ANP and ADH, and urine osmolality during night and day, and by investigating the differences in these variables among patients with different severity levels of OSA. In addition, given the expectation that CPAP therapy will result in normalization of intrathoracic pressures, and a subsequent restoration of the hormonal balance that determines nighttime urine concentrations, further studies should be undertaken to determine whether treatment of OSA improves nocturia, or more specifically, nocturnal polyuria.
This study has some limitations; the patients enrolled in the study were subjects who visited the urology outpatient clinic due to urologic problems that might contribute to nocturia besides OSA. Therefore, generalization of our results could be difficult since this kind of selection-bias was present in the study population. To assess the causal relationship between nocturia and sleep disturbance, future studies in larger and more homogenous groups of patients are needed.
In summary, this study emphasizes that nocturia can be a possible sleep disturbing factor and an independent predictor of self-reported insomnia. We conclude by emphasizing that nocturia could be one of the main causes of reduced sleep quality in adults and that it might be related to the presence of OSA. From the clinical point of view, nocturia might be useful as a screening tool for OSA, but further research is required on this topic.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.