Overlap Syndrome
Article information
Abstract
Chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) were common disorder that has been gaining increased attention as an important factor affecting health. The coexistence of COPD and OSA, which was termed overlap syndrome, is assumed to have affected many patients. The increases in morbidity or mortality are higher in patients with overlap syndrome than in patients with OSA or COPD alone. Nevertheless, the impact of OSA on COPD is not yet known. Patients with COPD experience sleep-related abnormalities, including sleep-disordered breathing associated with low sleep quality and hypoxia. Continuous positive airway pressure treatment accompanied by oxygen therapy has recently been attracting attention as the treatment of choice for overlap syndrome. Clinical consideration should be given to its potential sleep-related features that have not yet been recognized in order to improve clinical symptoms and quality of life for patients affected by overlap syndrome.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is one of the most prevalent chronic respiratory disorders worldwide. Timely treatment is particularly important, because COPD is highly associated with the quality of life, disability, and mortality of affected persons.1 Obstructive sleep apnea (OSA) is also a common disorder that has increasingly been gaining attention as an important factor affecting health, particularly because of its adverse effects on the cardiovascular system.2 Coexistence of COPD and OSA, which was termed overlap syndrome by David Flenley three decades ago, is assumed to have affected many patients.3 However, no clear mechanism of the association of COPD with OSA has yet been elucidated, such as whether COPD causes development of or predisposes individuals to OSA, or whether these two disorders have common causative factors.1
Definitions and Epidemiology
The definition of overlap syndrome as a state in which COPD and OSA are concomitant in an individual requires a review of several aspects, given that COPD and OSA can have different degrees of severity and require differential evaluations and treatments. COPD is characterized by persistent limitation in airflow with progressive aggravation, and is associated with enhanced chronic inflammatory response to the presence of particles and gases in the airway or lungs.4 The overall severity of COPD can be strongly influenced by exacerbation and co-morbidities in patients, one of which is OSA. COPD tends to be found in patients with chronic respiratory symptoms, such as dyspnea, cough, and sputum, and a history of exposure to risk factors such as smoking. The criteria for COPD diagnosis include the clinical symptoms just mentioned, and post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 0.70 by spirometry.5 Patients with OSA are typically affected by frequent pharyngeal collapse and temporary stoppage of breathing when falling asleep. The resulting apnea frequently induces hypoxia, CO2 retention, and awakening. OSA is generally defined as an apnea/hypopnea index (AHI) > 5 events/h. The presence or severity of OSA can be confirmed through sleep study.6,7 Using the status of FEV1/FVC < 0.7 plus FEV1 < 80% predicted as a reference standard, the prevalence of COPD worldwide is about 10–14% of the general population.8–10 Several studies have reported a sex-dependent prevalence of 20% in males and 9% in females.11,12 The Sleep Heart Health Study reported that 0.5% of the world population experiences airflow obstruction, while a European study on patients with mild COPD estimated the prevalence of OSA to be 3%.13 According to a recent European study, 78.1% of patients with COPD have night-time symptoms. Patients that experience night-time symptoms are more likely to experience daytime breathlessness and exacerbation of symptoms, and to have received drug treatments more frequently during the preceding year than patients who do not experience night-time symptoms.14
Clinical Features of the Overlap Syndrome
Chronic obstructive pulmonary disease symptoms, such as dyspnea, cough, and sputum, are direct causes of interrupted sleep. They are also associated with the sleep study findings that patients experiencing reduced total sleep time and sleep efficiency suffer from daytime hypersomnolence.15 Patients with COPD are subject to nocturnal oxygen desaturation and diurnal gas exchange abnormalities. Low partial pressure of oxygen (PaO2), in particular, is an indicator of nocturnal desaturation.16 Nevertheless, > 50% of patients with OSA experience significant desaturation during sleep, even without sleep apnea.17 Thus far, this phenomenon has been neglected, despite the increased mortality in affected patients. Although OSA is not more prevalent in patients with COPD than in those without, risk factors such as age, smoking, peripheral edema, and steroid use increase the prevalence of obstructive apnea.5 Obesity in patients with COPD is a key contributor to dyspnea during sleep,18 exacerbation of pulmonary arterial hypertension, and obesity hypoventilation syndrome. Such patients display clinical features similar to those in patients with blue-bloater type COPD. In contrast, patients with advanced COPD tend to lose weight, resulting in a decreased risk of upper airway obstruction.5 Nocturnal hypoxemia is one of the most important sleep abnormalities related to COPD and OSA.1 Overlap syndrome causes the nocturnal hypoxemia to be more severe than that induced by COPD or OSA alone.19 Hypoxic events are associated with increase in the systemic and pulmonary blood pressure and arrhythmias, which can result in cor pulmonale in chronic cases.20 Exacerbation of COPD and increase in OSA are directly associated with increased nocturnal death compared with non-COPD and non-OSA cases.21,22 Although comparable data on overlap syndrome are not available, Mc-Nicholas and Fitzgerald21 reported that patients with blue-bloater type COPD show a particularly high rate of nocturnal death. The so-called “blue bloaters” generally experience dyspnea during sleep.21,22 Taken together, these findings suggest that patients with overlap syndrome have higher mortality rates than those with COPD or OSA alone. Studies on patients with OSA have shown a higher risk of mortality in those with OSA and co-morbid COPD. Marin et al.23 reported that an untreated group with overlap syndrome had higher all-cause mortality than a COPD-only group (42.2% vs. 24.2%) after a median follow-up of > 9 years. Occurrence of nocturnal hypoxemia in patients with overlap syndrome can be explained by two factors. The Sleep Heart Health Study reported that patients with overlap syndrome have a higher risk for more severe nocturnal hypoxemia than those with OSA without co-morbid COPD. Furthermore, FEV1/FVC is a marker of continuous hypoxemia, and an indicator of elevated morbidity and mortality risks.19 Lavie et al.24 reported co-morbid COPD to confer a seven-fold increase in the risk of death in patients with OSA. One study reported a co-morbid OSA-induced increase of mortality in patients with COPD. Mermigkis et al.25 also showed that patients with overlap syndrome have significantly lower quality of life than those with COPD alone. Continuous hypoxia is presumably the causative factor of the elevated risk of mortality. In addition, nocturnal hypercapnia can be a major exacerbating factor in patients with overlap syndrome. McNicholas26 suggested that COPD and OSA are systemic disorders that can cause cardiovascular disease via various pathways, and that their pathogenesis may be influenced by oxidative stress and various inflammatory cytokines, such as tumor necrosis factor alpha, interleukin-6, and interleukin-8 (Fig. 1).
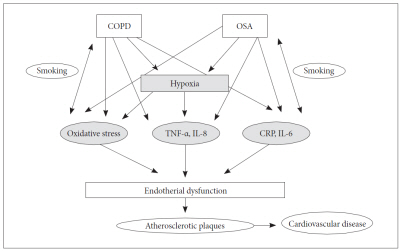
COPD and OSA are systemic disorders. COPD: chronic obstructive pulmonary disease, OSA: obstructive sleep apnea, TNF: tumor necrosis factor, IL: interleukin, CRP: C-reactive protein. Adopted from McNicholas WT. Am J Respir Crit Care Med 2009;180:692–700.
The majority of patients are initially diagnosed with either COPD or OSA, followed by differential evaluation by specialists. Patients with highly severe COPD often experience night-time symptoms, but the quality and pattern of sleep are rarely influenced in mild cases. Therefore, OSA- or sleep-related symptoms should be evaluated with polysomnography in the latter cases. The American Thoracic Society and European Respiratory Society jointly recommend overnight testing if a patient presents with symptoms of mild COPD and evidence of pulmonary hypertension.28 Patients with overlap syndrome and co-morbid pulmonary hypertension (OS-PH patients) tend to have relatively mild lung dysfunction or oxygenation compared to those with COPD only or co-morbid pulmonary hypertension (COPDPH patients). The FEV1 and FEV1/FVC of OS-PH patients are 1.8 L and 0.64, respectively, whereas the respective values in COPDPH patients are 1 L and 0.50. Furthermore, OS-PH patients are awakened at an average PaO2 of 64 mm Hg, whereas COPD-PH patients are awakened at 55 mm Hg. Flenley3 recommended a polysomnogram for patients with COPD whose SpO2 falls during sleep.1,19 Applying nocturnal oximetry alone for patients with COPD does not yield reliable diagnostic outcomes, because a fall in SpO2 during sleep may simply be ascribed to COPD, or in part, to overlap syndrome.1
Obtaining a detailed smoking and respiratory symptom history, followed by pulmonary function test, where applicable, is criticalin patients with OSA. Patients with OSA and diurnal hypoxemia or hypercapnia should undergo examination to check for co-morbid COPD. Moreover, focus on the diagnosis of overlap syndrome should be placed on evaluating pulmonary hypertension, by methods such as echocardiography or right-heart catheterization.1
Management
Treatment should aim to maintain adequate oxygenation without interruption and preventing sleep-disordered breathing.
Continuous Positive Airway Pressure
Continuous positive airway pressure (CPAP) is currently the standard treatment for OSA, and has increasingly been accepted as the standard treatment for overlap syndrome. However, CPAP alone is not adequate to completely correct hypoxia, which requires supplemental oxygen.1,29 Nevertheless, a consensus has not been reached regarding whether CPAP is effective for improving diurnal pulmonary function in patients with stable COPD. Arguments have also been made for off-loading the respiratory muscles to decrease hypoventilation, oxygen consumption, and production of carbon dioxide.30 Thus, muscles allowed to rest by the use of CPAP can become active to prevent increased upper-airway resistance that can occur during sleep.1 Otherwise, CPAP would cancel out the intrinsic positive end-expiratory pressure in severe cases of COPD.1 Mezzanotte et al.31 reported that use of CPAP in patients with COPD results in significant improvement on the 12-min walk test and maximum inspiratory force, with improvement of diurnal oxygenation and hypercapniaas well. Applying CPAP in patients with overlap syndrome has yielded contradictory results on pulmonary function testing. Several studies reported post-CPAP improvements in FEV1, PaO2, PaCO2, and mean pulmonary artery pressure.32 However, Toraldo et al.33 reported simultaneous weight loss, suggesting that the improvements arose from the weight loss. In contrast, O’Brien and Whitman34 reported in a retrospective study that CPAP can decrease pulmonary function. However, this result may have been biased, as most patients treated with CPAP have progressive disease and symptoms. In a Brazilian cohort study, Machado et al.35 confirmed overlap syndrome in 15% of patients with COPD referred to undergo long-term oxygen therapy, and reported that the oxygen therapy plus CPAP group had considerably higher 5-yr survival than the oxygen therapy-only group (71% vs. 26%). In a Spanish cohort study, Marin et al.23 stated that CPAP eliminated the additional OSA-induced mortality risk in patients with overlap syndrome compared to those with COPD only.
Noninvasive Ventilation
Noninvasive ventilation (NIV) has been attracting attention for patients with stable hypercapnic COPD.36 However, studies related to this topic have only been small-scale trials which yielded inconsistent results. Although positive airway pressure is accepted as a standard treatment, it is associated with discomfort in patients with overlap syndrome; thus, these patients may serve as ideal subjects for NIV, particularly because they are chronically hypercapnic. McEvoy et al.37 and Windisch et al.38 reported that NIV significantly reduces mortality. The former study reported a mortality-reducing effect of NIV in patients with hypercapnic-stable COPD, without showing any changes in pulmonary function or diurnal blood gases, whereas the latter study reported simultaneous improvements in both pulmonary function and diurnal blood gases. However, these studies were limited in that they were conducted in high-intensity NIV inpatient settings.37,38 The effects of bilevel PAP in patients with overlap syndrome have not been clearly determined. Further research is necessary to determine whether NIV is more effective than CPAP supplemented with oxygen therapy for the treatment of patients with overlap syndrome.
Other Managements
Weight loss and oxygen therapy
Weight loss is therapeutically beneficial for patients with OSA or obesity, but is associated with increased mortality in patients with COPD.39 Therefore, no reports have advocated weight loss for the treatment of overlap syndrome. However, a diet and exercise program may be advantageous for patients with mild COPD.1
Oxygen is the key factor for treating diurnal and nocturnal hypoxemia. Oxygen therapy provided at ≥ 18 h/day, including during sleeping hours, is effective for reducing mortality.40 Patients with COPD experiencing hypoxemia during sleep have elevated mortality. They maintain normal oxygen conditions during the day and their quality of sleep can be improved by correction of nocturnal hypoxemia, but no significant improvement in mortality has been observed.41,42 Few reports have been published on the positive effects of supplemental oxygen administration for patients with OSA. Despite mitigating the severity of nocturnal hypoxemia, no positive influence was observed on sleep patterns, awakening, or daytime somnolence.43 According to a study by Alford et al.44 on the effect of supplemental oxygen administration for the treatment of overlap syndrome, although nocturnal hypoxemia was improved, the duration of obstructive events increased from 25.7 to 31.4 seconds, resulting in an increase in PCO2 from 52.8 to 62.3 mm Hg and a decrease in pH. This result suggests that oxygen therapy should not be used as a single therapy for patients with overlap syndrome.44 The most serious consequence of hypoventilation during sleep is hypoxia. Adequate oxygen therapy plays a crucial role in treating various disorders associated with sleep-disordered breathing. The oxygen concentration supplied to minimize CO2 retention should be carefully regulated in accordance with PaO2; however, warnings regarding the harmful effects of CO2 retention in patients receiving oxygen therapy were exaggerated in the past. Numerous reports in recent years have confirmed that nocturnal oxygen therapy maintains moderate CO2 retention, and is usually non-progressing.45 High-flow oxygen administration is a low-risk treatment in cases of serious CO2 retention to maintain 90–92% SaO2 when COPD is exacerbated.46 Therefore, a sufficient oxygen supply for the maintenance of SaO2 > 90% should be assured during oxygen therapy; however, special care should be taken to avoid excess supply of oxygen. A nasal cannula is favored for the provision of nocturnal oxygen therapy over a face-mask because of the risk that the mask will slip off during sleep.47
Pharmacological therapy
The influences of conventional agents, such as bronchodilators and anti-inflammatory agents, should be considered for nocturnal drug administration in patients with COPD. Additionally, substances administered to improve sleep quality should not adversely influence ventilation or gas exchange.
Bronchodilator and anti-inflammatory agents
Cholinergic tone increases during the night in patients with COPD, contributing to aggravation of the airflow obstruction and gas exchange during sleep.1 One study reported a significant improvement in sleep quality and gas exchange in patients with COPD undergoing ipratropium treatment.48 Another study reported that tiotropium does not significantly improve sleep quality, but significantly increases nocturnal SaO2.49 Martin et al.48 investigated the effects of ipratropium in patients with moderate-to-severe COPD and reported improved nocturnal oxygen saturation, subjective sleep quality, and total hr of REM sleep. A study evaluating treatment with tiotropium showed that it did not influence sleep quality, but enhanced nocturnal SaO2, particularly during REM sleep, which is significant because REM sleep is when the most serious oxygen desaturation occurs.49 A study on treatment methods for sleep-disordered breathing in patients with COPD found the effects of beta-agonist therapy to be extremely limited. However, salmeterol improved nocturnal gas exchange at a similar level to that of tiotropium.50 Oral steroids contribute to preventing drop of the nocturnal oxygen concentration, while also increasing total sleep hours in patients with stable COPD.51 The results of studies on inhaled corticosteroids are not yet available, and it remains unclear whether COPD treatment improves OSA conditions in patients with overlap syndrome. Theophylline is an efficient treatment for patients with sleep-disordered breathing, as it stimulates the central respiratory system and diaphragmatic contractility.52 That it has the same effect on patients with COPD is presumably attributable to the decrease in trapped gas volume rather than to its bronchodilating effect. Results regarding the newer phosphodiesterase 4 agents have not yet been published.
Other medications
Almitrine has been used as a potent carotid body agonist to stimulate ventilation and improve pulmonary ventilation/perfusion. The overall effect of this hypoxia-reducing agent is advantageous for treating conditions related to nocturnal hypoxia, such as COPD. In particular, treatment was reported to improve nocturnal SaO2 during REM sleep, as compared to the placebo group. However, this agent is no longer used because of reports of side effects, which include pulmonary hypertension, dyspnea, and peripheral neuropathy.53 Studies have also been conducted on tricyclic antidepressants and selective serotonin reuptake inhibitors in relation to sleep-disordered breathing in patients with COPD. These agents fragment REM sleep, thereby reducing the severity of oxygen desaturation typical of REM sleep. However, they are rarely used in the treatment of COPD-related sleep disorders, as they show only short-term benefits associated with nocturnal SaO2 in patients with COPD. Benzodiazepine and non-benzodiazepine hypnotics shorten sleep latency and improve sleep efficiency, thus reducing the frequency of awakening. However, they have been observed to have adverse effects on ventilation, including hypoventilation associated with hypoxemia and hypercapnia.54 In addition, because of their effects of reducing the awakening response to hypercapnia and increasing the frequency of sleep apnea, they should not be administered to patients with highly severe COPD. Substances such as zolpidem can be administered to patients with less-severe COPD without serious side effects.55 Melatonin receptor antagonists shorten sleep latency while improving sleep efficiency.56 They have been reported not to exert adverse effects on the frequency of sleep apnea or PaO2 in patients with COPD.
CONCLUSION
The number of patients with overlap syndrome is assumed to be high, as OSA and COPD are both common diseases. The increase in morbidity and mortality is higher in patients with overlap syndrome than in those with OSA or COPD alone. Nevertheless, the impact of OSA on COPD is not yet known. Patients with COPD experience sleep-related abnormalities, including sleep-disordered breathing, associated with low sleep quality and hypoxia. CPAP treatment accompanied by oxygen therapy has recently been attracting attention as the treatment of choice for patients with overlap syndrome. Clinical consideration should be given to its potential sleep-related features that have not been recognized to improve the clinical symptoms and quality of life of patients affected by overlap syndrome.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.