Effects of Continuous Positive Airway Pressure on White Matter Microstructure in Patients With Obstructive Sleep Apnea
Article information
Abstract
Background and Objective
Obstructive sleep apnea (OSA) has significant effects on quality of life and may lead to cognitive impairments. Continuous positive airway pressure (CPAP) is the standard treatment for OSA and has been shown to improve sleep disturbances and daytime dysfunction. In this study, we aimed to assess the effects of CPAP on white matter (WM) integrity using longitudinal diffusion tensor imaging (DTI) tests.
Methods
Twenty-two male patients with moderate to severe OSA were recruited, and thepatients underwent DTI scanning before and 6–44 months after CPAP treatment. Sixteen male patients with untreated OSA who were not compliant with CPAP were included as a reference group. We compared the functional anisotropy (FA) values between baseline and follow-up magnetic resonance imaging in both the CPAP and untreated groups using tract-specific statistical analysis (TSSA) method.
Results
The TSSA analysis showed that FA values in the middle part of the right corticospinal tract were increased after treatment in the CPAP group. In the untreated group, no significant change in FA value was observed between baseline and follow-up. In the CPAP group, the post-treatment FA value in the anterior part of the right anterior thalamic radiation was significantly correlated with the duration of CPAP therapy, after controlling for age, body mass index, and baseline FA value.
Conclusions
Our study suggests that long-term CPAP treatment could gradually reverse OSA-induced injury to the WM microstructure, particularly WM associated with the motor and limbic systems. The study findings provide new insights into the mechanisms of cognitive improvement after CPAP treatment in patients with OSA.
INTRODUCTION
Obstructive sleep apnea (OSA) is one of the most prevalent sleep disorders, and is characterized by repetitive interruption of breathing during sleep due to complete or partial airway obstruction. Repetitive apneas and hypopneas cause intermittent hypoxemia, hypercapnia, microarousals, and fragmented sleep [1], resulting in structural and functional alterations in the brain. OSA is associated with cognitive impairments in various domains, including memory, executive function, and attention [2,3]. Several neuroimaging studies obtained using various modalities have investigated the pathophysiological mechanisms of OSA to identify loss of gray matter (GM) volume [4,5], disruption of white matter (WM) integrity [6,7], limited cerebral blood flow [8,9], and alterations in functional connectivity [10,11].
Continuous positive airway pressure (CPAP) has been the most effective and widely used treatment for OSA. Studies have shown that CPAP treatment eliminates the respiratory disturbances and sleep fragmentation [12], and also reduces the risk of cardiovascular diseases [13-15]. Furthermore, CPAP has been shown to improve subjective daytime functioning and cognitive performance [16-18]. Although investigations into the effects of CPAP through brain imaging are underway, most studies have been limited to perfusion imaging and functional magnetic resonance imaging (MRI). It has yet to be determined whether this functional improvement (dynamic change) results in recovery of the structural damage that occurs in OSA. While earlier voxel-based morphometry studies found no significant longitudinal GM changes after CPAP therapy [19,20], other groups demonstrated improvement in GM volume [21,22], especially after long-term treatment [23].
Studies focusing on the effects of CPAP on WM structures are limited. The first study that used a diffusion tensor imaging (DTI) technique found normalization of functional anisotropy (FA) after 12 months of CPAP, but the number of OSA patients was small [24]. A recent study investigated changes in WM microstructure after CPAP treatment along with cerebral perfusion changes, but only six patients were included, and their duration of treatment was short (six weeks) [25]. In regard to DTI analysis methods, both studies used a voxel-wise approach, which cannot specify anatomically which WM tracts are impaired.
Tract-specific statistical analysis (TSSA), a method that was developed and validated by our group, improves the mapping of tract diffusion coefficients along the corresponding major anatomical tracts [26]. This system uses the results of subject-specific tractography and a tract classification method that acquires the fiber directions in subject-specific tractography maps. Using the TSSA method, it is able to identify local disruptions in specific fiber tracts using the orientation of all fiber tracts, which provides clues to clarify functional abnormalities. This process has demonstrated clinical utility in sleep disorders to identify tract-specific abnormalities in patients with OSA, narcolepsy, and restless legs syndrome [7,27,28].
In the present study, we used the TSSA method to investigate the effects of CPAP treatment on the WM integrity of patients with OSA by comparing longitudinal changes on DTI imaging in a treated group and an untreated group.
METHOD
Participants
Twenty-two male patients with moderate to severe OSA (apnea– hypopnea index [AHI] ≥ 15) were recruited from the Sleep Clinic at Samsung Medical Center in Seoul, South Korea. All patients underwent MRI scans within one month prior to initiating CPAP treatment (pre-CPAP scan). They were scanned once more after treatment (post-CPAP scan, mean treatment duration: 21.5 months, range: 6–44 months). All subjects were adherent to CPAP therapy during the entire study period, which was defined as ≥ 4 hours nightly usage for at least 70% of nights. As reference participants, we recruited 16 untreated OSA patients with failure in using, or refusal to use, CPAP treatments. They underwent follow-up MRI scans 12–62 months after their baseline MRI scans. Exclusion criteria for both the CPAP treatment group and the untreated group (reference participants) were as follows: 1) comorbid heart and respiratory diseases; 2) history of malignancy; 3) history of cerebrovascular or neurological disease (neurodegenerative diseases, epilepsy, head injury); and 4) alcohol abuse, illicit drug abuse, or current intake of psychoactive medications.
The Institutional Review Board of Samsung Medical Center (IRB No. 2020-06-015) approved the study protocol, and written informed consent was obtained from all participants. The methods were carried out in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines.
MRI Scanning
The T1- and diffusion-weighted images were acquired from all participants using the same 3.0-T MRI scanner (Philips 3.0 T Achieva; Andover, MA, USA). The T1-weighted images were obtained using the following scanning variables: 0.5 mm sagittal slice thickness, over contiguous slices with 50% overlap, no gap, a repetition time (TR) of 9.9 ms, an echo time (TE) of 4.6 ms, a flip angle of 8°, and a matrix size of 240 × 240 pxl. The images were reconstructed to 480 × 480 pxl over a 240 mm field of view. In the whole-brain diffusion-weighted MRI examination, sets of axial diffusion-weighted single-shot echo-planar images were collected with the following parameters: 128 × 128 pxl acquisition matrix, 1.72 mm × 1.72 mm × 2 mm voxels, 70 axial slices, a 220 mm × 220 mm field of view, a TE of 60 ms, a TR of 7385 ms, a flip angle of 90°, a slice gap of 0 mm, and a b-factor of 600 s/mm2. For baseline images without diffusion weighting (the reference volume), diffusion-weighted images were acquired from 45 directions. All axial sections were acquired parallel to the anterior commissure–posterior commissure line.
Image Processing and Statistical Analysis
The T1 and DTI image processing and statistical analysis are all included in the TSSA method developed by our group. The TSSA method consists of three stages: 1) extracting the seven major anatomic tracts from the diffusion coefficients obtained from deterministic tractography, 2) generating a tract profile, and 3) performing statistical analysis. An explanation of the TSSA method was previously provided [7,26,27]. Fig. 1 shows the key components of each stage in the TSSA method.
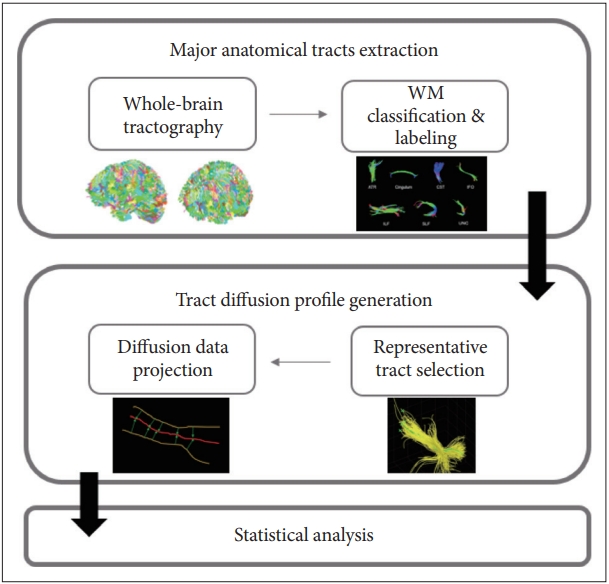
An overview of the white matter (WM) tract-based approach of the tract-specific statistical analysis.
First, tremendous fiber tracts are derived by deterministic tractography after skull stripping and Eddy current distortion correction as DWI common preprocessing. The FA values were calculated simultaneously. Then, the fiber tracts were automatically grouped and labeled into seven major tracts of the anterior thalamic radiation (ATR), cingulum, corticospinal tract (CST), inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus, and uncinate fasciculus by multi-atlas-based clustering and labeling method, while also considering the individual variability of shape and position [29].
Second, representative streamlines were selected using a fiber density map after removing outlier fibers to compute a tract profile of the seven major tracts. Because the representative streamline is a fiber that exists in a location through which most of the major tracts pass, the selected representative tract contains the maximum density. Next, all fiber tract FA values were projected to the representative values of corresponding major tracts. To project these FA values, point matching between other fiber tracts and the representative fiber tract was preceded by an optimal point matching method [30], which is robust to spatial distortion and variability. The projected FA values were averaged with weights based on the Mahalanobis distance.
As the final step, statistical analysis of the FA values in the CPAP treatment groups and the untreated group was performed. To evaluate the changes in diffusion parameters along the tract over time for each group, a permutation-based analysis of covariance was applied with 10000 permutations. A cluster-based statistics (CBS) method that is widely used in voxel-based morphometry was applied for multiple comparisons correction [31,32]. To investigate the association between FA value after CPAP therapy and duration of CPAP therapy in the treatment group, permutation-based tests for correlation were performed. After that, the significance level of the correlation coefficient was adjusted using CBS [33]. Partial correlation coefficients were calculated for the follow-up FA values in the tract profile after controlling for age, body mass index (BMI), AHI, and baseline FA value as covariates. The TSSA method was performed with a combination of the Diffusion Toolkit [34], FMRIB Software Library (FSL) [35], MATLAB R2022a (MathWorks, Inc., Natick, MA, USA), and our in-house code.
RESULTS
Baseline Characteristics
Table 1 summarizes the detailed clinical characteristics and polysomnographic (PSG) findings between the CPAP group and the untreated group. The demographic characteristics and PSG parameters were not significantly different between the two groups. The time intervals between baseline and the follow-up MRI scans were longer in the untreated group than in the CPAP group (41.0 ± 15.4 vs. 21.5 ± 20.4 months, p < 0.001).
Comparisons of TSSA Results Between the CPAP and Untreated Groups
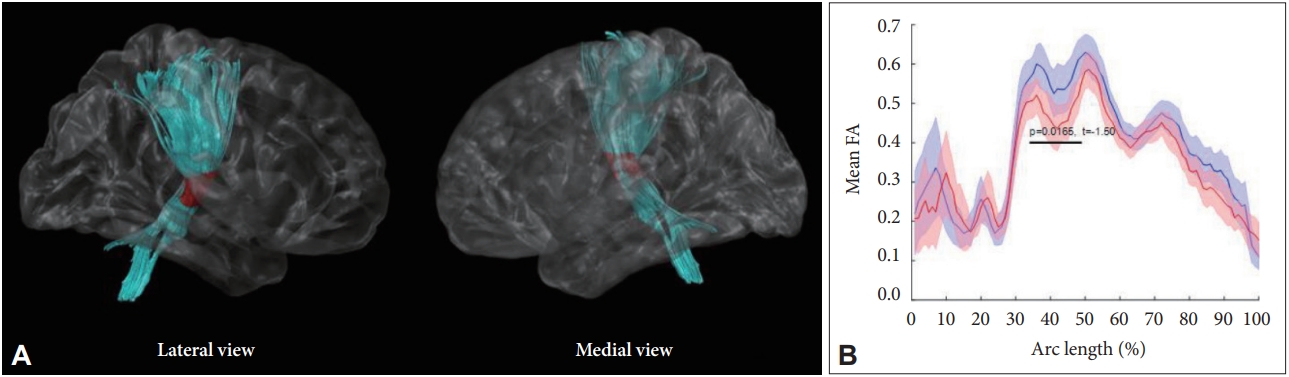
In the CPAP group, the mean FA value in the middle part of the right CST was significantly increased after treatment, after controlling for age and BMI (p = 0.017) (Fig. 2). The untreated group did not show any significant change in the FA value on the follow-up MRI scans, compared to baseline. Supplementary Table 1 (in the online-only Data Supplement) presents detailed FA values of the seven major WM tracts at baseline and on follow-up MRI.
Association Between TSA Values and Durations of CPAP Therapy
For the relationships between tract-specific FA values and durations of CPAP therapy in the treatment group (n = 22), the FA value in the anterior part of the right ATR after CPAP treatment was positively correlated with the duration of CPAP therapy, after controlling for age, BMI, and baseline FA value (r = 0.250, p = 0.034) (Fig. 3). The correlation between the FA value of the right ATR and treatment duration were significant even after controlling the effect of baseline AHI in addition to abovementioned factors (r = 0.250, p = 0.037).
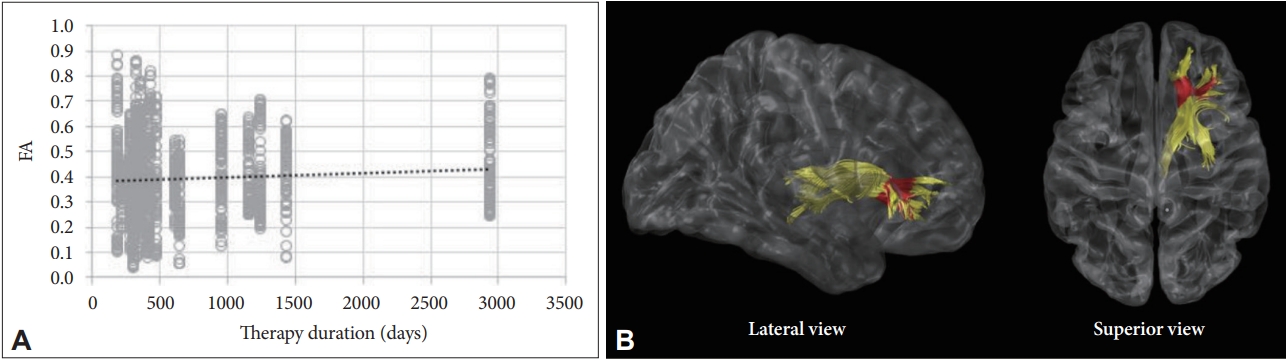
Correlation analysis with adjustments for age, body mass index, baseline FA values between post-treatment FA value, and the duration of CPAP therapy. (A) FA value in the right anterior thalamic radiation was positively correlated with the duration of CPAP therapy. (B) Red colors indicate the portions of fiber tracts where FA value significantly correlated with the treatment duration. FA, functional anisotropy; CPAP, continuous positive airway pressure.
DISCUSSION
This is the first study to demonstrate “tract-specific” WM changes after CPAP treatment by comparing longitudinal DTI scans of untreated and CPAP-treated OSA patients. We found an increase in FA value of the right CST after CPAP treatment, while there was no significant change in the FA values of the untreated group. Furthermore, we found significant correlation between the duration of CPAP treatment and post-treatment FA values of the right ATR in the CPAP group. The TSSA method enabled location of the specific portion and direction of WM fiber changes, which helps clarify the CPAP effect on brain function.
The CST, which showed significant FA changes after CPAP in our study, is a major neural tract known to convey sensorimotor information. Decreased FA values in the CST were observed in patients with OSA in previous studies [6,36], and one of them revealed a correlation between FA value in the CST and the severity of OSA [36]. This finding implies that the microstructural integrity of the CST is affected by the detrimental process of OSA, and our study demonstrated that CPAP could alleviate the disruption in the CST. Previous DTI studies that have investigated WM changes after CPAP treatment did not detect a significant difference in this area. Castronovo et al. [24] observed changes in FA and MD in the parietal and prefrontal regions, while Maresky et al. [25] found increases in FA in the hippocampus, medial temporal lobe, fusiform gyrus, and parietal lobule [24,25]. These conflicting findings could be explained by the large number of participants and their long-term treatment in our study, which enabled more sensitive detection of the treatment effect of CPAP.
The duration of CPAP treatment is an important factor for WM recovery. Castronovo et al. [24] found limited changes in WM after three months of CPAP, whereas an almost complete reversal of WM abnormalities was observed after 12 months of CPAP. Our study found a positive correlation between posttreatment FA value in the right ATR and the duration of treatment in the range of 6–44 months. Our study and that of Castronovo et al. [24] imply that WM recovery in response to CPAP treatment is a gradual process over a long period of time. Although the underlying pathomechanism is unclear, a decrease in reactive oxygen species production due to a reversal of hypoxia and normalized cerebral vasculature could allow activation of the intrinsic mechanisms of axonal repair [25,37]. From a clinical perspective, our findings suggest that future DTI studies to investigate the effects of CPAP would be better to include patients treated for at least six months, and highlight the importance of long-term adherence to CPAP treatment for OSA patients.
The ATR consists of fibers between the mediodorsal thalamic nuclei and the frontal cortex, and between the anterior thalamic nuclei and the anterior cingulate cortices. The role of the anterior thalamic nucleus in cognition as part of the limbic system is well known, and destruction of the ATR has been reported to be related to cognitive decline in various conditions [38-40]. Our team previously reported decreased FA value in the right ATR in male OSA patients [7]. In the present study, we did not find a significant change in FA value in the right ATR in our direct comparison between pre- and post-CPAP treatment. However, a positive correlation between post-treatment FA in the right ATR and the duration of CPAP implies that the microstructure of ATR was gradually affected by CPAP treatment. This may further explain the cognitive improvement after CPAP treatment in previous studies [16,17].
The limitations and strengths of this study should be addressed. First, the duration of CPAP treatment varied in participants from 6 to 44 months. The time interval between baseline and follow-up scans was longer in the untreated group than in the CPAP group, although we corrected it as a covariate in our statistical analysis. Second, we did not assess the vascular risk factors of the participants, such as hypertension and diabetes, which could affect SM integrity. Third, in regard to the imaging analysis method, we only investigated seven major tracts, because of the restrictions of example data used in the tract segmentation method. Lastly, since we did not perform post-treatment PSG, changes in apnea severity or objective sleep quality before and after treatment could not be assessed. Despite these limitations, this study has several strong points. Most importantly, we adopted a new analysis method that specified WM tract abnormalities to assess the treatment effect of CPAP. Furthermore, the number of participants included in this study was far larger than the number in previous DTI studies that investigated the effects of CPAP, which adds strength to our findings. Last, we included patients who had been treated for a long-term period (mean duration: 21.5 months), which enabled the long-term effect of CPAP on WM microstructure to be revealed, as well as the correlation between treatment duration and WM integrity to be identified.
In conclusion, the present study supports the evidence that microstructural WM abnormalities observed in OSA can change with treatment. Our TSSA methods used in this study revealed that the treatment effect was particularly prominent for tracts involved in the motor and limbic systems. Furthermore, our findings emphasize the necessity for long-term use of CPAP in brain structural recovery. This longitudinal study provides novel insight into the pathophysiology underlying OSA and the brain structural recovery process under CPAP treatment.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.17241/smr.2022.01459.
Comparison of adherence by manager and device
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Authors’ Contribution
Conceptualization: Eun Yeon Joo, Hea Ree Park. Formal analysis: Hye Ryun Kim. Funding acquisition: Hea Ree Park. Investigation: Eun Yeon Joo, Hea Ree Park. Methodology: Joon-Kyung Seong. Project administration: Eun Yeon Joo. Resources: Eun Yeon Joo. Software: Joon-Kyung Seong. Supervision: Eun Yeon Joo, Joon-Kyung Seong. Validation: Eun Yeon Joo. Visualization: Hye Ryun Kim. Writing—original draft: Hea Ree Park, Hye Ryun Kim. Writing—review & editing: Eun Yeon Joo, Joon-Kyung Seong.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Funding Statement
This work was supported by a grant from the research program of the Korean Society of Sleep Medicine, 2021, a National Research Foundation of Korea (NRF) grant, funded by the Korea government (MSIT) (No. NRF-2020R1C1C1014725 and No. 2021R1G1A1008471), and Samsung Medical Center Grant (OTC1190671).