Habitual Subjective Sleep Continuity is Not Associated With Fluid Intelligence: An Exploratory Study
Article information
Abstract
The link between sleep and cognition is well-established, but the link between subjective sleep and fluid intelligence is poorly understood. The aim of this exploratory study was to examine the relationship between habitual subjective sleep continuity and fluid intelligence. In this study, a total of 56 healthy sleepers (Mage = 30.91 years; SDage = 12.93 years) completed two fluid intelligence (abstract reasoning and two-dimensional mental rotation) tasks after completing seven consecutive days of sleep diaries. Relationships between subjective sleep continuity (total sleep time [TST]; sleep efficiency [SE%]); wake after sleep onset (WASO) and sleep onset latency (SOL), and task accuracy and speed were assessed using Pearson correlations. Overall, there were no associations between subjective sleep continuity (TST, SE%, WASO, SOL) and either task accuracy or speed (adjusted p-values > 0.0125). Overall, habitual subjective sleep continuity and fluid intelligence may not be associated. These results should be replicated in larger samples.
INTRODUCTION
A sufficient quantity of high-quality sleep is necessary to maintain good physical, psychological and brain health. Sleep and cognition are very closely linked, as sleep plays an important role in memory and cognitive processing [1], and an insufficient sleep duration and quality negatively affects multiple cognitive domains including sustained attention, memory and decision-making [2,3]. Although there is a well-established association between sleep and cognition, the link between sleep and intelligence, which refers to the construct of general cognitive ability, is less clear [4,5].
In particular, relatively little is known regarding the link between subjective sleep and fluid intelligence. Fluid intelligence refers to the important cognitive ability to reason, learn from experience, identify complex patterns and solve novel problems; this is in contrast to crystallised intelligence, which instead reflects previously-acquired knowledge [6,7]. Fluid intelligence is relatively stable across the lifespan, but typically declines rapidly as a function of ageing, and alterations to fluid intelligence are potentially a feature of neurodegenerative dementia [8]. Therefore, if subjective sleep continuity and fluid intelligence are associated, modifications to sleep continuity might maintain fluid intelligence later in life. There is potentially a common underlying link between sleep and fluid intelligence, as fluid intelligence appears to be underpinned by activity in the locus coeruleus, which is the major noradrenergic system and a neural region which has a key role in sleep and wake regulation [7,9].
There is an apparent link between subjective sleep and fluid intelligence: it has been observed that subjectively-measured sleep quality was positively associated with fluid intelligence, where higher sleep quality was associated with greater objectively-measured reasoning performance [10]. However, to date, no studies have examined the relationship between specific aspects of subjective sleep continuity, which can be easily, cheaply and reliably measured using subjective sleep diaries [11], and fluid intelligence. Therefore, the primary aim of this study was to assess the relationship between specific aspects of subjective sleep continuity which represent subjective sleep duration and quality (total sleep time [TST] and sleep efficiency [SE%]), and two closely-related aspects of fluid intelligence: abstract reasoning and mental rotation [12]. It was hypothesised that subjective TST and SE would be positively associated with 1) abstract reasoning accuracy, 2) mental rotation accuracy, and 3) the mental rotation response speed to correct answers.
METHODS
Participants
A total of 73 healthy sleeper participants (Mage = 30.64 years, SDage = 12.45 years) were recruited from the staff and student population of Northumbria University. This sample size was based on an a priori power analysis, conducted using G*Power 3.1 [13], which indicated that a minimum of 29 participants were required on the basis of an expected medium effect size (r = 0.5 at 80% power).
Individuals were eligible to participate if they were: 1) aged ≥ 18 years and 2) self-reported healthy good sleepers. Participants were not eligible if they had a current self-reported sleep disorder (e.g., insomnia or sleep apnea) or subjective sleep problems, or if they had a history of sleep disorders/sleep problems. Participants provided informed consent and the study was approved by Northumbria University Faculty of Health and Life Sciences Ethics Committee (no. 27960). Participants were not renumerated.
Measures
In order to assess habitual subjective sleep quality, participants completed the Pittsburgh Sleep Quality Index (PSQI) [14]. Consensus Sleep Diaries (CSD-M) [15] were used to assess measures of habitual subjective sleep continuity, including TST; time in bed (TIB), SE%; calculated as (TST/TIB × 100); sleep onset latency (SOL); the number of awakenings (NWAK) and wake after sleep onset (WASO).
Procedure
The study was delivered online using Qualtrics (Provo, UT, USA) and the PsyToolkit platform (www.psytoolkit.org [16]). After providing informed consent at baseline (day 0), participants completed the PSQI, PHQ-9, and Generalised Anxiety Disorder Questionnaire. On each subsequent morning (day 1 to day 7), participants completed the CSD-M. On day 7, participants completed two fluid intelligence tasks and were subsequently debriefed.
Fluid Intelligence Tasks
Participants completed two fluid intelligence tasks: the 12-item short form version of the Raven’s Standard Progressive Matrices (RSPM-SF) [17] task, which is a problem-solving task that becomes progressively more difficult over time, and a mental rotation task (MRT) [18], which requires participants to match two-dimensional line drawings of three-dimensional block figures to a target stimulus; in order to complete the task, participants must mentally rotate the target stimulus. The RSPM-SF has similar levels of validity and reliability to the full 60-item RSPM [17].
Statistical Analyses
To assess RSPM-SF task accuracy, the percentage of correct answers were derived from this task. To assess MRT performance, the percentage of correct answers, and the reaction time (RT) to correct answers (expressed in milliseconds [ms]), were derived as markers of accuracy and speed, respectively. Additionally, the coefficient of variation (COV%) of mean RTs to correct answers was derived; the COV% is a marker of intra-individual variability in response time and was calculated as (SDRT / MRT) × 100.
To assess if sleep continuity was associated with abstract reasoning accuracy, and mental rotation accuracy and speed, separate Pearson correlations were conducted between 1) TST, SE%, WASO, SOL and RSPM-SF percentage of correct answers; 2) TST, SE, WASO, SOL, and MRT percentage of correct answers, and: 3) TST, SE%, WASO, SOL and the MRT RT to correct answers. Participants with a minimum of five completed sleep diary days (n = 61) were included in subsequent analyses [19]. All p-values were adjusted for multiple comparisons (adjusted p-value = 0.0125).
Two additional analyses examined if sleep continuity was associated with the intra-individual variation in MRT responses to correct answers (COV%; adjusted p-value = 0.0125), if PSQI scores were associated with RSPM-SF or MRT percentage of correct answers (adjusted p-value = 0.025). Finally, we examined if good (n = 40) or poor (n = 21) sleepers, defined on the basis of the PSQI, differed in task performance using between-groups t-tests adjusted for multiple comparisons (adjusted p-value = 0.025).
RESULTS
Complete RSPM-SF data were obtained from 56 participants (Mage = 30.91 years; SDage = 12.93 years) and complete MRT data were obtained from 31 participants (Mage = 25.49 years; SDage = 9.02 years). The main reason for MRT non-completion was participant attrition. Demographic and relevant questionnaire results are shown in Supplementary Table 1 (in the online-only Data Supplement).
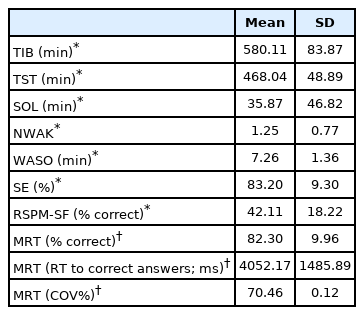
Summary sleep diary information, and task performance, is summarised in Table 1. There were no significant relationships between TST, SE%, WASO, SOL, and RSPM-SF accuracy (all p-values > 0.0125) (Table 2). Similarly, there was no significant association between TST, SE%, WASO and SOL and MRT accuracy, or the response to MRT correct answers (p-values > 0.0125). There was no association between subjective sleep continuity or MRT COV% values (p-values > 0.0125), and PSQI scores were not associated with RSPM-SF or MRT accuracy (r = 0.05 and r = -0.06, respectively; p-values > 0.025). Good and poor sleepers did not differ with respect to task performance (all p-values > 0.025).
DISCUSSION
The aim of the present study was to examine the relationship between specific aspects of subjective sleep continuity (TST and SE%) and fluid intelligence, assessed using abstract reasoning and mental rotation tasks. Unexpectedly, there was no relationship between subjective sleep continuity and abstract reasoning accuracy, or between subjective sleep continuity and mental rotation accuracy or response speed. These results indicate that habitual subjective sleep continuity is not associated with two closely-related aspects of fluid intelligence: abstract reasoning or mental rotation. Whilst these results are surprising given the very close link between sleep, memory, and cognition [1-3], to our knowledge, this is the first study to specifically examine the relationship between habitual subjective sleep continuity, as derived from sleep diaries, and fluid intelligence.
One possible reason for the unexpected findings observed in the present study is that there may only be a relationship between objective sleep macrostructure and fluid intelligence, and not between subjective sleep continuity and fluid intelligence. Specifically, the association between objective sleep macrostructure and fluid intelligence is likely to be predominantly underpinned by sleep spindles, which are distinctive patterns of waxing and waning neural oscillations occurring during non-rapid eye movement (NREM) objective sleep [6]. This is demonstrated by the fact that a range of previous studies have observed that fluid intelligence is associated with objective sleep spindle density and amplitude, in adolescents and adults, and that this relationship is also observed during daytime nap opportunities [6,20-22]. Sleep spindles originate from thalamocortical regions [23]; these neural regions have also been shown to be involved in fluid intelligence [24]. A further explanation for the present findings is that the relationship between habitual subjective sleep continuity and fluid intelligence may be affected by the relative instability of night-to-night subjective sleep, and differences in intra-individual subjective sleep. It is known that subjective sleep continuity can be affected by a wide range of factors, including genetics, social behaviours, sleep attitudes, environment and timing, both within and between individuals [25]; therefore, future studies should specifically examine the impact of nightly variations in subjective sleep continuity upon fluid intelligence.
This study could be extended by focussing on habitual objective sleep. As it is very likely that only objective sleep macrostructure is associated with fluid intelligence, ambulatory polysomnography (PSG) could be utilised to measure habitual objective sleep, rather than subjective sleep continuity, over an extended period of time in a home environment. Ambulatory PSG is reliable, cost-effective, and recent work has demonstrated that participants can also accurately self-collect PSG information [26]. Future studies could also administer intelligence tasks on a daily basis in order to examine whether short-term (i.e., nightly) changes to sleep continuity may influence fluid intelligence performance, measured on the subsequent morning.
The main strength of the present study is in the good level of ecological validity. One particular limitation is in the small sample size. Whilst although the associations between intelligence and objective sleep macrostructure, and particularly fast sleep spindle amplitude, which is potentially the most reliable marker of intelligence, are consistent, they are generally small with only modest effect sizes [4]. Despite the potential involvement of the locus coeruleus in fluid intelligence [7,9], this may also explain why we did not observe a relationship between habitual sleep quality, measured using the PSQI, and fluid intelligence. One previous study, which found that higher PSQI-assessed subjective sleep quality was associated with fluid intelligence, did so with a sample size of approximately 12000 participants [10]; therefore, associations between subjective sleep continuity and fluid intelligence may only be apparent in extremely large sample sizes.
Further limitations of the present study include the high levels of participant attrition, and the use of self-report sleep assessments, as these may be affected by response bias [11]. Although healthy self-reported sleepers were recruited, we could not verify that this was the case and future studies may wish to use clinical interviews in screening; however, global PSQI scores were within the expected range for good sleepers (< 5) [14]. In relation to this point, future studies with larger sample sizes may wish to specifically examine differences between good and poor sleepers; this may provide an insight into the potential casual role of subjective sleep in fluid intelligence. Similarly, this study could be replicated in poor sleepers only. Additionally, in the present study, we were unable to examine if men and women showed different patterns of association between subjective sleep and fluid intelligence. This is relevant as, for example, one previous study observed that in females, intelligence and fast sleep spindle amplitude were positively associated, but that in males, intelligence and fast spindle density was negatively related [27]. However, it was beyond the scope of the present study to examine if this was the case; therefore, future work could examine if biological sex differences underpin this relationship. Similarly, given the potential influence of age upon fluid intelligence [8], specific age groups (e.g., young adults) could be investigated in future.
Overall, the results of the present study indicate that there appears to be no relationship between habitual subjective sleep continuity and fluid intelligence. These results should be replicated using larger sample sizes.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.17241/smr.2022.01522.
Participant demographics (n = 56)
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Authors’ Contribution
Conceptualization: Emily L. Jensen, Greg J. Elder. Data curation: Emily L. Jensen, Greg J. Elder. Formal analysis: Emily L. Jensen, Greg J. Elder. Investigation: Emily L. Jensen. Methodology: Emily L. Jensen, Greg J. Elder. Project administration: Emily L. Jensen. Resources: all authors. Software: Emily L. Jensen. Supervision: Greg J. Elder. Validation: Nayantara Santhi, Greg J. Elder. Writing—original draft: Emily L. Jensen. Writing—review & editing: all authors.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Funding Statement
This study was supported by Northumbria University.
Acknowledgements
We would like to thank all study participants.