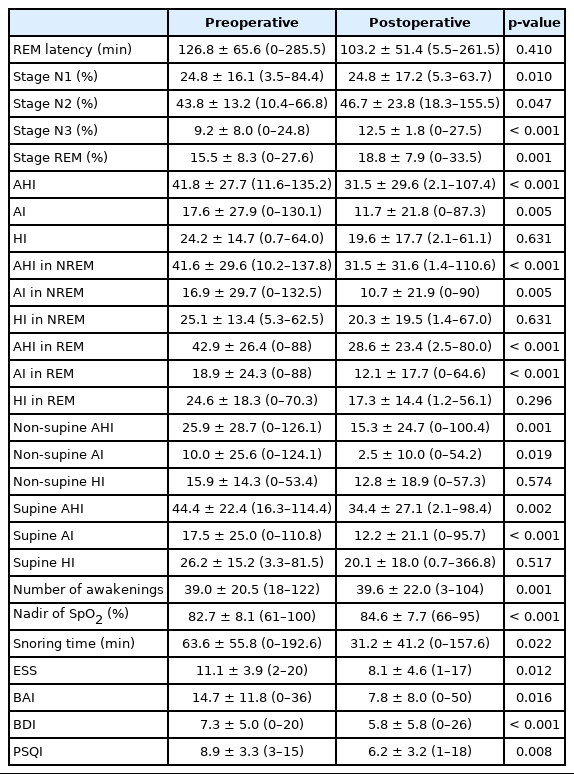
Therapeutic Effect of Extended Uvulopalatal Flap Surgery in Patients With Obstructive Sleep Apnea
Article information
Abstract
Background and Objective
Although numerous studies have reported surgical results of uvulopalatopharyngoplasty, efficacies of extended uvulopalatal flap (EUPF) surgery have only been reported in a few studies. Thus, the aim of this study was to evaluate the success rate of EUPF and investigate the advantage and practicality of this surgery.
Methods
Medical records of patients who underwent EUPF surgery were retrospectively analyzed. Through medical record analysis, demographic information was confirmed. Postoperative polysomnography (PSG) results were divided into a surgical ‘success’ group and a ‘failure’ group. Patients’ PSG results, questionnaires, and cephalometry were comparatively analyzed.
Results
All sleep parameters of PSG except rapid eye movement latency and hypopnea index were significantly improved after surgery. Ten patients were in the success group (success rate, 33.3%) and 20 patients were in the failure group. Among preoperative PSG parameters, apnea-hypopnea index, apnea index, and number of awakenings showed significant differences between the two groups. However, there were no significant differences in results of cephalometry or Friedman stage between the two groups.
Conclusions
The EUPF surgery can change sleep factors and improve subjective symptoms in obstructive sleep apnea patients. It could be considered as one of the treatment options for patients with surgical indications, although its success rate was only 33.3%.
INTRODUCTION
The importance of treatment for adult obstructive sleep apnea (OSA) has recently been emphasized because OSA has various side effects such as cardiovascular diseases, daytime sleepiness, and metabolic disorders [1,2]. In order to diagnose OSA, a physical examination is performed along with patient’s history taking and questionnaire, which are subjective evaluation methods. In addition, a flexible laryngoscopy or drug-induced sleep endoscopy (DISE) can be used to confirm the site of upper airway obstruction [3,4]. Although cephalometric analysis can be used for objective evaluation, the most important diagnostic method is polysomnography (PSG) [1,5]. Treatment modalities for adult OSA can be mainly divided into surgical and non-surgical treatments such as drugs, oral appliances, and continuous positive airway pressure (CPAP) [1,6-9]. The treatment method for a patient is carefully decided by considering the upper airway obstruction site, the severity of the disease, the presence of comorbidities, the compliance with CPAP use, and the patient’s social requirements [10-15]. As the primary goal of surgical treatment is to widen the area of the upper airway that causes apnea during sleep, the operation range should be determined according to the extent of obstruction area and the obstruction site.
When the oropharynx causes OSA, the uvulopalatopharyngoplasty (UPPP) devised and published by Fujita et al. [16] in 1981 is often used. After performing bilateral tonsillectomy and making an incision along the glossopalatal arch, UPPP widens the palate by dissecting submucosal tissues of the soft palate together with the uvula. It pulls the posterior pillar of the tonsil forward and causes it to be fixed on the upper side. However, UPPP can cause complications such as postoperative edema, pain, and velopharyngeal incompetence (VPI) due to deformation of the soft palate [17-19]. To alleviate these side effects, Powell et al. [20] announced the uvulopalatal flap in 1996 and Li et al. [21] introduced the extended uvulopalatal flap (EUPF) in 2003. Although many studies have been conducted and published on results of surgery after UPPP, only a few studies on efficacies of EUPF surgery both in South Korea and abroad have been reported. Thus, the aim of this study was to evaluate the success rate of EUPF and investigate the advantage and practicality of this surgery.
METHODS
Participants
From January 1, 2018 to December 31, 2021, medical records of patients who underwent EUPF surgery at the Department of Otorhinolaryngology-Head and Neck Surgery, Chungnam National University Hospital (CNUH) with PSG before and after surgery were retrospectively analyzed. Of a total of 71 patients, 30 completed preoperative and postoperative PSG. Through medical record review, demographic information including patient’s sex and age, type of surgery, body mass index (BMI, kg/m2), follow-up period, PSG results, questionnaires, comorbidities, and complications were obtained. Friedman stage was measured by confirming the patient’s tonsil size as well as Friedman palate position through physical examination [22]. This study was conducted after obtaining approval from the Institutional Review Board (IRB) of CNUH (IRB File No. 2022-06-004).
Surgical Procedure
Extended uvulopalatal flap
All patients were operated by the same surgeon under general anesthesia. After performing bilateral tonsillectomy, incisions were added to superior-laterally from the anterior pillar to the upper pole of tonsillar fossa. After designing the uvulopalatal flap with a pen, the mucosa and submucosal adipose tissue were dissected and removed while preserving the muscle layer using a cold knife and an electrosurgical pencil (Electrosurgical Pencils with EdgeTM Coated Electrodes; Medtronic, Minneapolis, MN, USA). If the uvula was redundant or elongated, the uvular was partially resected at this stage. The uvulopalatal flap was lifted in the soft palate direction and sutured using 3-0 coated VICRYL® (Ethicon Inc., Cornelia, GA, USA) and Monosyn® (B.Braun, Barcelona, Spain). The anterior pillar, superior constrictor muscle, and posterior pillar were sutured together 2–3 times using VICRYL® (Fig. 1).
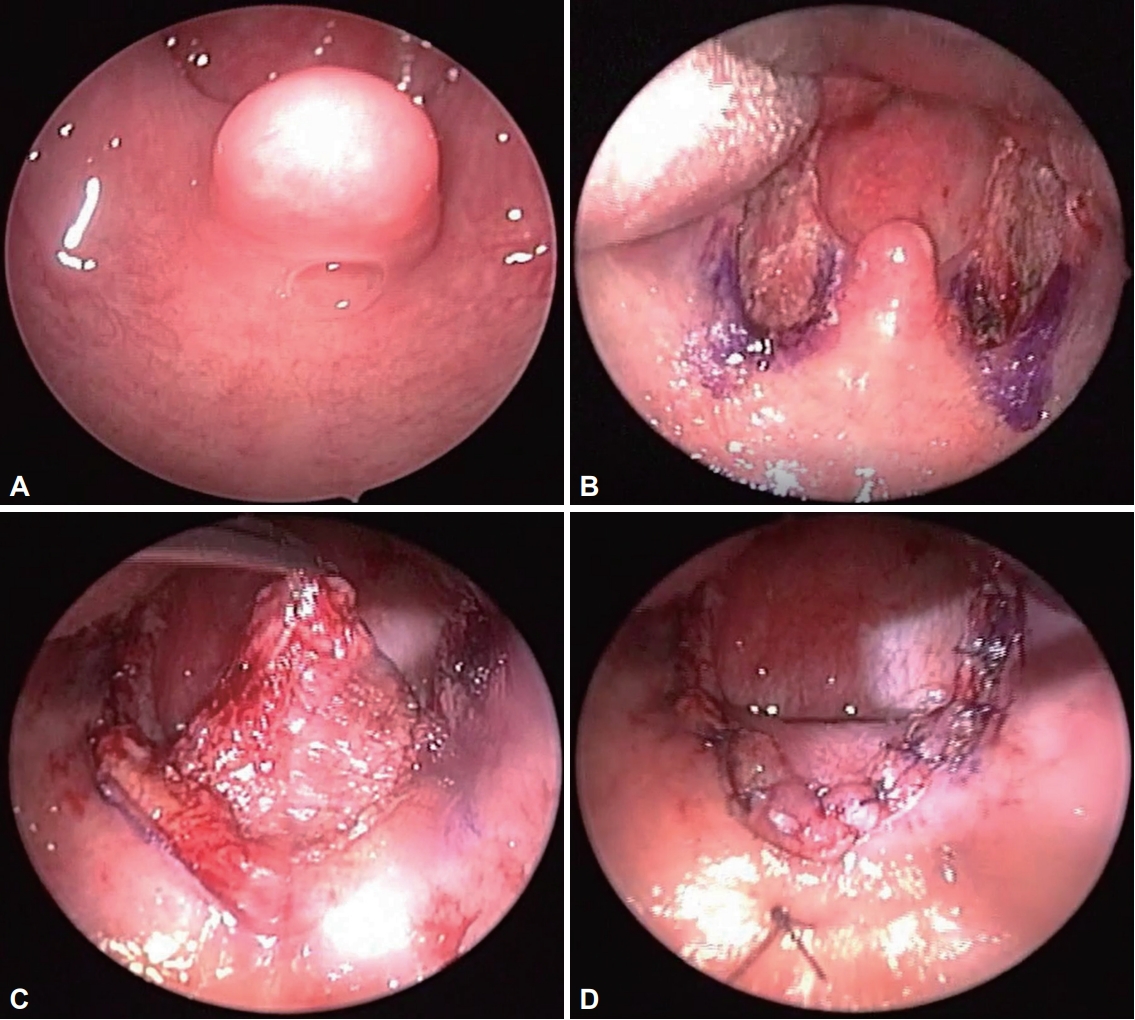
Extended uvulopalatal flap surgery. A: Soft palate and uvula before surgery. B: After bilateral tonsillectomy. C: A submucosal flap of the soft palate was removed by extending the incision on the upper pole of the tonsils on both sides. D: The uvula is folded over the soft palate and fixed with sutures.
Septoturbinoplasty and tongue base radiofrequency volume reduction
All patients underwent a physical examination including a Müller maneuver before surgery. An objective evaluation was performed with acoustic rhinometry and rhinomanometry for symptoms such as nasal obstruction. A single skilled surgeon performed all operations. Osteomeatal unit computed tomography (OMU CT) was taken as needed. Patients with chronic rhinosinusitis (CRS) or chronic rhinosinusitis with nasal polyp underwent endoscopic sinus surgery at the same time. In addition, when inferior turbinate hypertrophy and tongue base obstruction were observed, radiofrequency turbinate volume reduction and tongue base radiofrequency volume reduction (TBRVR) were performed, respectively, using COBLATOR®II (Arthro-Care, Austin, TX, USA) [23].
Polysomnography
All participating patients underwent full-night level I PSG at CNUH before and after surgery. Index values of PSG outcomes were classified according to the definition of the scoring manual version 2.6 of the American Academy of Sleep Medicine published in 2020. Criteria for surgical success were: 1) a decrease of more than 50% of the previous apnea-hypopnea index (AHI) and 2) AHI less than 20.
Questionnaires
Epworth Sleepiness Scale
The Epworth Sleepiness Scale (ESS) score was used to check weekly symptoms due to OSA. The ESS consisted of a total of 8 questions. For each question, a 4-point score (from 0 to 3) was obtained [24]. The total score was 24 points, with a score of 0–5, 6–10, 11–12, 13–15, and 16–24 indicating lower, higher, mild, moderate, and severe excessive daytime sleepiness, respectively.
Beck Anxiety Inventory
The Beck Anxiety Inventory (BAI) consisted of 21 items with multiple-choice to determine the severity of a patient’s anxiety [25]. In this study, the Korean version of the BAI was used [26]. For each question, patient’s answer had a 4-point score (from 0 to 3), with a total score of 0–7, 8–15, 16–25, and 26–63 indicating minimal, mild, moderate, and severe anxiety, respectively.
Beck Depression Inventory
The 21-item Beck Depression Inventory (BDI) is mainly used to measure the severity of depression [27]. The Korean version of the BDI [26] was used in this study. Each item had a 4-point score from 0 to 3, with a total score of 0–7, 8–15, 16–25, and 26–63 indicating minimal, mild, moderate, and severe depression, respectively.
Pittsburgh Sleep Quality Index
Pittsburgh Sleep Quality Index (PSQI) is a method in which patients self-evaluate their sleep quality over the past month [28]. It had seven components with a total of 19 items. The total score ranged from 0 to 21 to reflect the sleep quality and quantity, with a higher score indicating a poorer sleep quality.
Cephalometry
Cephalometric analysis was used to evaluate musculoskeletal abnormalities and soft tissue characteristics of the patient’s face. Lateral cephalometry was taken before surgery for all patients in a sitting position while holding their breath. All measurements included in the results were measured manually by one surgeon using the same landmarks. Variables included posterior airway space (PAS), distance from the mandibular plane to the hyoid bone (MP-H), length of the soft palate, thickness of the soft palate, length of the maxilla, and length of the mandible. The PAS diameter was measured from the extension line passing through the gonion and the supramental. The length of the soft palate was calculated from the posterior nasal spine to the tip of the soft palate. The maxillary length was measured as the distance from the anterior nasal spine to the posterior nasal spine. The mandibular length was calculated as the distance from the gnathion to the gonion [5,29,30].
Statistical Analysis
All statistics were performed with the Statistical Package for Social Sciences (SPSS; IBM Co., Armonk, NY, USA) version 22.0. Statistics were compared with an independent sample t-test. χ2 test was used to compare the distribution of Friedman stages according to success of the operation. When the p-value was less than 0.05, it was defined as a statistically significant case.
RESULTS
Demographics
Thirty patients (1 female and 29 males) had a mean age of 40.27 years (range, 18 to 62 years). Their mean BMI was 28.34 kg/m2. AHI was observed at 42.88 events per hour before surgery. Regarding both tonsil size, size 3 (36.7%) was the most common. Nine patients had size 1 and size 2, respectively. The Friedman palate position was position III in 19 (63.3%) patients. It was position II in the rest. Friedman stage was stage II in 17 patients, stage III in 11 patients, and stage I in two other patients. As for patients’ comorbidities, four patients had hypertension, which had the highest number of patients. Two patients were being treated for diabetes and one patient was being monitored for ischemic heart disease and arrhythmia. Only one patient had both diabetes and hypertension (Table 1). One patient was taking anxiolytics and antidepressants for major depressive disorder. One patient was taking antidepressants and atypical antipsychotic medications for insomnia.
Surgical Method
None of the 30 patients underwent EUPF alone. Twelve (40.0%) patients underwent septoturbinoplasty with EUPF. Seven patients underwent bilateral ESS surgery, EUPF and septoturbinoplasty, and five patients underwent EUPF and turbinoplasty. Patients with tongue base obstruction confirmed through physical examination underwent TBRVR. Three of four patients who received TBRVR underwent septoturbinoplasty simultaneously (Table 2). For all patients who underwent ESS, the range of surgery was determined according to the OMU CT taken before surgery. All patients underwent bilateral surgery.
Comparison of Polysomnography before and after Extended Uvulopalatal Flap Surgery
For follow-up, PSG was performed at an average of 6.9 (range, 2–36 months) months after surgery (Table 3). All sleep parameters of PSG except rapid eye movement (REM) latency and hypopnea index (HI) were significantly improved after surgery. Mean AHI regardless of REM or non-rapid eye movement (NREM) or position was also significantly improved mainly due to improved apnea index (AI) rather than HI. Snoring time also showed a definite decrease from 63.63 minutes to 31.18 minutes (p = 0.022). Oxygen saturation was also improved (p < 0.001). Scores to all four scores of questionnaires (ESS, BAI, BDI, and PSQI) conducted to examine patient’s subjective symptoms were all significantly reduced.
Comparison of Preoperative Polysomnography Results between ‘Success’ and ‘Failure’ Groups
Patients were divided into ‘success’ and ‘failure’ groups according to the definition of successful operation (AHI decreased by more than 50% and AHI < 20). Ten patients were assigned into the success group based on this definition, while 20 patients were assigned into the failure group (Table 4). Preoperative PSG was compared between the success group and the failure group. Among preoperative PSG parameters, AHI (p = 0.049), AI (p = 0.027) (regardless of REM or NREM sleep), and number of awakening (p = 0.032) were significantly lower in the success group than the failure group. However, mean AHI and AI calculated according to body position (supine and non-supine) did not shown significant differences between the two groups.
Comparison of Cephalometric Parameters and Demographic Factors between Success and Failure Groups
Cephalometric and demographic factors such as PAS diameter, MP-H distance, soft palate length, soft palate thickness, maxillary length, mandibular length, age, BMI, and Friedman stage were compared between success and failure groups. However, there were no significant differences in these factors between the two groups (Table 5).
Additional Treatment after Extended Uvulopalatal Flap Surgery
If a patient had an abnormality in the PSG performed after surgery, treatment was carried out after sufficient conversation with the patient. After surgery, the mean follow-up period was 9.27 months (range, 2–35 months). In the case of residual AHI, six patients wore CPAP or auto-titrating positive airway pressure. None of them used a mandibular advancement device. A total of 12 patients received conservative treatment, of which ten patients attempted to lose weight. The remaining two patients decided to proceed with positional therapy during sleep. Of the total 30 patients, eight (26.67%) complained of postoperative complications. Five patients complained of foreign body sensation. One patient complained of difficulty in eating due to VPI. Two patients had pain at the surgical site. Complications of all eight patients improved with conservative treatment. These patients are being monitored at the outpatient clinic.
DISCUSSION
It is difficult to know the exact surgical success rate because the criteria for surgical success in OSA patients are defined differently depending on the author. The results of surgery such as surgical success rate are affected over time [31]. In the case of UPPP, which has relatively more published surgical results than EUPF, Choi et al. [32] have stated that one of the critical factors in predicting the success of UPPP is the Friedman stage. The lower the stage, the higher the success rate. According to Friedman et al. [22], when the criteria of surgical success are considered as respiratory disturbance index (RDI) when patients are classified according to the Friedman stage, the surgical success rate is the highest at 80.6% in stage I and the lowest at 8.1% in stage III. Li et al. [33] have compared UPPP results with OSA severity using AHI with Friedman stage. The overall surgical success rate was 78% which was different from the overall surgical success rate of 90% in mild OSA or 74% in severe OSA, although these differences were not statistically significant. In the anatomy-based stage, the surgical success rate decreased from 100% in stage I to 96%, 65%, and 20% in stage II, III, and IV, respectively. Also, the change in AHI showed significant correlations with Friedman tongue position and tonsil size.
On the other hand, since EUPF was introduced in 2003, few papers have announced the surgical success rate for EUPF so far. In a previous study, the success rate of EUPF was 82% when considering only the decrease in RDI regardless of Friedman stage in OSA patients [34]. To our knowledge, this is the first paper to analyze surgical results for EUPF in Korean patients. Although the surgical success rate in this study was low (10 out of 30 patients), it was found that all sleep-related factors improved after surgery. Interestingly, it was found that AI significantly improved after surgery than HI during AHI regardless of REM sleep or sleeping posture. As the arousal decreased significantly along with the overall AHI decrease while deep sleep and REM sleep increased significantly, it was found that EUPF surgery improved airway obstruction in patients, which contributed to the improvement of sleep quality. In addition, it was found that ESS and PSQI scores as subjective indicators of sleep quality improved significantly after surgery.
DISE is generally performed for OSA patients preoperatively. Multilevel surgery is then performed according to the occluded area. It is known to have a high postoperative success rate. In this study, if possible, physical examination, including a DISE test, was performed before surgery. Septoturbinoplasty and TBRVR were operated together if obstruction of the nasal cavity and hypopharynx was expected. The surgical period of patients participating in this study was when positive airway pressure (PAP) was prescribed as the primary treatment for OSA patients as it was applied to medical insurance in South Korea. We tried to include patients who failed to comply with PAP and wanted surgery on their own, patients who did not show tongue base obstruction in the preoperative Müller maneuver, and relatively young patients. Therefore, in this study, the number of patients who underwent tongue base volume reduction was small, unlike other previous studies.
Braga et al. [35] have compared results of cephalometric analysis of OSA patients by dividing them into surgical success and surgical failure groups. The criterion for surgical success was patients with postoperative AHI less than 5 with postoperative AHI decreasing by more than 50% compared to preoperatively. When the two groups were compared, there were no significant differences in results of cephalometry. Similarly, in this study, the criteria for surgical success were different (AHI < 20 events/hour and AHI decreased by more than 50% compared to that before surgery). However, there were no significant differences in cephalometric variables between the two patient groups according to the success of surgery. Some studies have shown that MP-H and PAS are related to OSA [32]. Ryan et al. [36] have defined the ‘response’ of surgery as when the postoperative AI is less than four events/hour or decreased to 60% or less compared to the preoperative AI. After UPPP surgery, responders had a statistically significantly narrower PAS (p < 0.0005) and a significantly smaller ratio of PAS to tongue length (p < 0.001).
There were no cases of complications during hospitalization after surgery. Among the eight patients who developed complications, five patients complained of foreign body sensation as the most common symptom. The other patients had pain at the surgical site and VPI. However, all resolved without additional treatment. No severe complications were found.
A limitation of this study was that the number of patients participating in the study was small. This was because among patients diagnosed with OSA, not only the number of patients eligible for surgery was small, but also the number of patients with preoperative and postoperative PSG among patients who underwent EUPF was small. Second, because this study analyzed medical records retrospectively, results of DISE were insufficient and could not be added to the analysis. Finally, when analyzing cephalometry, although the same surgeon had the same reference point and measured it using the same program, bias could still occur due to characteristics of cephalometric radiographs. This is because X-ray film is a two-dimensional image, making it difficult to confirm shadows on soft tissues.
In conclusion, this is the first study that compares and analyzes surgical success rate and treatment effect before and after surgery in Korean patients undergoing an EUPF surgery. When diagnosed through PSG, EUPF surgery changed the rate of REM sleep and made significant differences in AHI, snoring time, and minimum oxygen saturation. It also resulted in a significant change in AI along with a change in AHI regarding success of the operation. Therefore, EUPF surgery can change sleep factors and improve subjective symptoms in OSA patients. It can be considered as one of the treatment options for patients with surgical indications in the future.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: Jae-Yoon Kang, Yong Min Kim. Data curation: MiRa Choi, Se Yeon Im. Formal analysis: Mi-Ra Choi. Funding acquisition: Yong Min Kim. Investigation: Se Yeon Im, Han Wool John Sung. Methodology: Se Yeon Im, Yong Min Kim, Soo Kyoung Park. Project administration: Yong Min Kim. Resources: Yong Min Kim. Software: Se Yeon Im. Supervision: Yong Min Kim. Validation: Yong Min Kim. Visualization: Se Yeon Im, Jae-Yoon Kang. Writing—original draft: Jae-Yoon Kang, Yong Min Kim. Writing—review & editing: Jae-Yoon Kang, Yong Min Kim.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Funding Statement
This work was supported by research fund of Chungnam National University.