Effect of Atypical Antipsychotic Medications on the Risk of Obstructive Sleep Apnea in Individuals With Schizophrenia
Article information
Abstract
Background and Objective
Weight gain and obesity could be side effects of taking atypical antipsychotic medications. They are major risk factors for obstructive sleep apnea. The purpose of this study was to investigate effects of atypical antipsychotic medications on the risk of obstructive sleep apnea in individuals with schizophrenia and control groups.
Methods
In this cross-sectional study, 123 patients with schizophrenia and 107 participants were selected from hospital staff and students using purposive sampling. Data were collected using structured clinical interview and Berlin questionnaires.
Results
Results showed that the risk of obstructive sleep apnea was significantly higher in individuals with schizophrenia. Age, body mass index, and phases of schizophrenia showed meaningful relationships with the duration of taking atypical antipsychotic medications with the risk of obstructive sleep apnea. However, the type of atypical antipsychotic medications did not have a significant relationship with the risk of obstructive sleep apnea.
Conclusions
Patients with schizophrenia treated with atypical antipsychotic medicationshave a higher risk of obstructive sleep apnea.
INTRODUCTION
Schizophrenia, the most common idiopathic psychotic disorder, is diagnosed based on psychiatric history and mental status examination [1]. The median age at the onset of schizophrenia in early adolescence is between 20 and 34 years of age. Its treatment involves hospitalization, administration of atypical antipsychotic medications (AAs), and psychological interventions [2,3]. AAs were introduced for treating psychopathological symptoms of disorders such as schizophrenia and emotional disorders. Although AAs are recommended as the first line treatment for individuals with schizophrenia and widely prescribed by physicians to treat psychosis, they have many side effects, including hormonal changes, extrapyramidal complications, and weight gain [4].
Main risk factors for AAs includes man gender, old age, large neck circumference, postmenopausal, excessive weight, body mass index (BMI), anatomical problems. Minor risk factors for AAs include smoking, obesity, and glucose intolerance [2,4-6]. Review and meta-analysis studies have reported that risk factors such as obesity, smoking, glucose intolerance and obstructive sleep apnea (OSA) are more prevalent in individuals with schizophrenia than in the general public [6-11]. OSA can cause depression, daytime drowsiness, and cognitive disorders, all of which often disrupt lives of schizophrenia patients. It might be mistaken as early negative symptoms [12].
Although advances have been made in the use of AAs in clinical psychiatry, there are increasing concerns about their use and severe medical complications [5]. Side effects of AAs are affected by several factors, including type, dosage, and the patient’s underlying response to AAs. The most common side effects include drowsiness and metabolic problems such as hyperglycemia, dyslipidemia, and substantial weight gain. Among side effects of AAs, obesity and metabolic changes play a major role in causing serious medical problems in the general population, including cardiovascular diseases, respiratory disorders, and OSA. OSA is a common respiratory disorder with negative effects on patient’s health and quality of life. OSA is characterized by frequent collapse of the pharyngeal airway. It affects 2% to 4% of the population [5]. However, using AAs is likely to cause weight gain and exacerbate OSA due to their potential effects on the upper respiratory function. Many studies have reported the prevalence of sleep disorders in individuals with schizophrenia [6,11,13]. However, effects of AAs on the risk of OSA remain unclear, needing more evaluation. Despite the methodological deficiencies of advantages and disadvantages AAs for patients with schizophrenia, AAs makes dysfunction on the onset and sleep maintenance.
Limited studies have evaluated the risk of OSA in patients with schizophrenia based on the type of AAs, duration of taking AAs, and phase of schizophrenia. Conducting studies about effects of AAs on the risk of OSA in individuals with schizophrenia can lead to a greater understanding about their side effects and early diagnosis and treatment of OSA of the clinician, which in turn can reduce the risk for cardiovascular diseases, cognitive disorders, and depression, thus dramatically improving patient’s quality of life.
Objectives
To compare risk of OSA in patients with schizophrenia and those in the control group based on important risk factors (age and BMI). Also, compare the effect of AAs on OSA in individuals with schizophrenia based on the type of AAs, compare the effect of AAs on OSA in individuals with schizophrenia based on phases of schizophrenia, and compare the effect of AAs on OSA in individuals with schizophrenia based on the duration of taking the AAs.
METHODS
Study Design and Participants
In this cross-sectional study, 123 patients with schizophrenia were selected with a purposive sampling method from psychiatric wards of Farabi Hospital, Kermanshah, Iran. The control group was selected from the hospital’s staff and students who did not receive neuroleptics. They were matched in terms of some demographic variables with schizophrenia patients. Patients with schizophrenia were selected according to the following study inclusion criteria: 1) older than 18 years of age, 2) having Diagnostic and statistical manual of mental disorders, 4th ed, Text Revision (DSM-IV-TR) diagnostic criteria for schizophrenia, 3) having no underlying severe cardiovascular, pulmonary, or renal diseases, 4) having no alcohol consumption, and 5) having no oral or maxillofacial anatomic problems. Patients with schizophrenia were divided into four groups according to the AAs used: olanzapine (n = 39), risperidone (n = 42), quetiapine (n = 20), and perphenazine (n = 22). The purpose of dividing schizophrenia into these four groups was to determine which type of AAs could affect the severity of sleep apnea in patients. Schizophrenia patients and the control group were matched based on important risk factors for OSA such as age, gender, BMI, and neck circumference (NC). This study was conducted in accordance with the declaration of Helsinki. It was approved by the ethics committee of Kermanshah University of Medical Sciences (clinical trial code: IR.KUMS.REC.1396.586). All participants completed the informed consent form before participating in this study.
Data Collection
Demographic questionnaire
This questionnaire was used to collect demographic data such as gender, age, BMI, NC, and medication use.
Structured clinical interview
Structured Clinical Interview for DSM-IV Axis I Disorders is a semi-structured interview based on diagnostic criteria of DSM-IV-TR. This scale was used to assess psychiatric disorders. Psychometric properties of this scale have been confirmed [14].
Berlin questionnaire
Berlin questionnaire is commonly used in sleep research and medicine to identify sleep respiratory problems. This tool can avoid unnecessary polysomnography to diagnose OSA in the general population. This is a 10-item questionnaire in three categories. The risk of sleep respiratory problems is considered low when none or one category is positive according to the total score. The risk is considered high when two or more categories are positive. Psychometric properties of this questionnaire have been confirmed [15].
Statistical Analysis
Data were analyzed using descriptive statistics (mean and standard deviation) and inferential statistics (independent t-test, odds ratio [OR], one-way analysis of variance, and Scheffe post hoc) in SPSS-18 software (IBM Co., Armonk, NY, USA).
RESULTS
Table 1 presents demographic variables of the study groups in terms of age, BMI, NC, medication use, phase of schizophrenia, and AAs. Results from t-test showed no significant differences in age, BMI, and NC (p > 0.05) between the two groups.
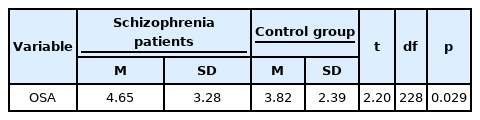
Results of t-test showed a significant difference in the risk of OSA between schizophrenia patients and the control group. In other words, the risk of OSA was significantly higher in the patients with schizophrenia than the control group (t = 2.20, df = 228, p = 0.029) (Table 2).
Result showed the risk of OSA was higher in schizophrenia patients treated with AAs than in the control group (OR = 0.906, 95% confidence interval = 0.827–0.992) (Table 3).
The analysis of variance test showed significant differences in the risk of OSA according to age (f = 7.27, p = 0.001), BMI (f = 38.91, p = 0.001), phases of schizophrenia (f = 8.73, p = 0.001), and duration of taking AAs (f = 9.16, p = 0.001) between patients with schizophrenia and the control group. However, there was no significant difference in the risk of OSA between patients with schizophrenia and the control group according to the type of AAs used (f = 0.16, p = 0.926) (Table 4).
Results of Scheffe post hoc test showed that participants were divided into three age groups: 29–40, 41–50, and 51–65 years. The risk of OSA in the age group of 50–41 years was higher than in the age group of 40–29, but there was no significant difference between the risk of OSA in the age group of 40–29 with 51–65 and 50–41 with 65–51 years. Results also showed that the risk of OSA increased steadily with increasing BMI. There was a meaningful difference in the risk of OSA between those with a normal weight and those with obesity (p < 0.001) and between those who were overweight and those with obesity (p < 0.001). However, there was no meaningful difference in the risk of OSA between normal weight and overweight groups (p > 0.05). Results also showed a significant difference in the risk of OSA between Prodromal and Residual phases (p < 0.001) and between active and residual phases (p < 0.001). However, there was no meaningful difference in the risk of OSA between prodromal and active phases (p > 0.05). With increasing duration of taking AAs, the risk of OSA also increased, showing a significant difference between 1–10 and 11–20 or 21–32 years. However, the risk of OSA showed no significant difference between 11–20 and 32–31 years (Table 5).
DISCUSSION
The present study mainly aimed to investigate the effect of AAs on the risk of OSA in patients with schizophrenia. Results showed that the risk of OSA was significantly higher in schizophrenia patients than in the control group. Results of OR analysis showed that the risk of OSA was 0.906% higher in schizophrenia patients than in the control group. This result agrees with results obtained from previous studies about effects of AAs on OSA [7,13-16]. For instance, Khazaie et al. [17] and Rishi et al. [18] have compared the severity of OSA in patients taking AAs to that in the control group. After matching the two groups in terms of age, gender, BMI, and NC, it was found that the risk of OSA was significantly higher in patients with schizophrenia using AAs. Although results of Drummond [19], Haupt and Newcomer [8], and Allison et al. [7] suggested a possible relationship between AAs and the presence or severity of OSA, an increased risk of OSA might be due to glucose intolerance caused by AAs rather than the inherent use of AAs.
Another aim of the present study was to investigate the relationship between OSA in patients with schizophrenia and the control group based on important risk factors (age, BMI, type of AAs, phases of schizophrenia, and duration of taking AAs). Results showed that the risk of OSA was increased with increasing age, although the risk was no longer significantly increases in the older age group. This result was in line with the study of Ancoli-Israel et al. [20], which showed the prevalence of OSA in older patients with schizophrenia was similar to that in healthy individuals, although schizophrenia patients have more severe OSA than healthy individuals. Shirani et al. [5] have shown no significant difference in the risk of OSA according to age of patients with psychiatric disorders.
The results also showed no significant difference in the risk of OSA in patients with overweight compared to normal weight, but the risk of OSA in patients with obesity was higher than normal weight and overweight. Patients with schizophrenia who are under long-term treatment with AAs might have an increased risk of OSA due to weight gain. However, regardless of the effect of AAs on BMI, various psychoactive medications have direct effects on sleep-related respiratory function. For example, anxiolytic and hypnotic medications from benzodiazepine family might exacerbate OSA due to central respiratory depression or pharyngeal muscle relaxation, while antidepressants might mildly reduce the severity of OSA by facilitating serotonin transmission [16,21]. Khazaie et al. [17] have also shown that AAs medications can cause weight gain and recognized as the main cause of OSA. There is a weight-independent association between AAs medications and worsening respiration during sleep. Results from some studies also indicate that using AAs may cause the risk of OSA, irrespective of weight gain [5,18,22-24]. Furthermore, Rishi et al. [18] have shown that the odds ratio of OSA in patients treated with AAs is 1.9. They concluded that AAs might cause OSA independently of weight or neck length.
The present study also aimed to compare increased risk of OSA in patients with schizophrenia in terms of type of AAs. In agreement with the results from James et al. [25], our results showed no significant relationship between AAs medications and the risk of OSA in patients with schizophrenia in terms of the type of AAs medication taken. James et al. [25] have shown no associations of AAs with high risk of OSA. Results of the present study also showed a significant difference in the risk of OSA between prodromal and residual phases and between active and residual phases. In agreement with results from previous studies, Yung and McGorry [26] have interviewed 21 patients. All of them suffered sleep disturbances during the prodromal period. Gourzis et al. [27] have reported that 21% of schizophrenia patients show sleep disorder in their first episode of psychosis. Iyer et al. [28] have stated that 65% of schizophrenia patients experience sleep disturbance during the prodromal period. Miller et al. [29] have found that 37% of patients experience ‘moderate’ to ‘extreme’ sleep disturbance in the prodromal phase of schizophrenia.
In conclusion, the present study showed more prevalent risk of OSA among individuals with schizophrenia treated with AAs compared to that in healthy controls based on Berlin questionnaire. Further research with more sample size is necessary to describe the usefulness of population-specific and objective OSA screening tools for assessing associations between AAs medications and OSA and for evaluating any benefits of treating OSA with continuous positive airway pressure on patient’s symptoms.
Limitations of the Present Study Include
Results for the present study should be interpreted with caution as it was a cross-sectional study. All participants were clinically suspected of sleep apnea. In other words, in the present study, the risk of sleep apnea among participants was assessed using data from the Berlin questionnaire. It would have been better to use objective tools such as polysomnography.
In the present study, findings of the Berlin questionnaire showed that sleep apnea was significantly higher in individuals with schizophrenia than in the control group. This finding was not investigated with polysomnography.
Since the present study was a cross-sectional study with a control group, only individuals with schizophrenia treated with AAs were selected. Thus, their results cannot be generalized to untreated individuals with schizophrenia.
Given the changing nature of the respiratory disorder, the inherent limitation in OSA diagnosis needs to be considered as a challenge for the reliability of this study and similar studies. Therefore, conclusions about the relationship between OSA and psychiatric disorders should be interpreted with caution. Given the above limitations and non-random selection of participants, results of the present study might not be generalized to other patients.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: Habibolah Khazaie, Aliakbar Parvizifard. Data curation: Siamand Mazhar, Sepideh Khazaie. Formal analysis: Arash Parsa Moghadam. Funding acquisition: Habibolah Khazaie. Investigation: Siamand Mazhar, Arash Parsa Moghadam, Sepideh Khazaie. Methodology: Mohammad Rasoul Ghadami, Arash Parsa Moghadam. Project administration: Habibolah Khazaie, Aliakbar Parvizifard. Resources: Habibolah Khazaie, Arash Parsa Moghadam, Siamand Mazhar. Software: Arash Parsa Moghadam. Supervision: Habibolah Khazaie. Validation: Habibolah Khazaie, Aliakbar Parvizifard. Visualization: Siamand Mazhar. Writing—original draft: Aliakbar Parvizifard, Arash Parsa Moghadam. Writing—review & editing: Arash Parsa Moghadam, Habibolah Khazaie, Aliakbar Parvizifard.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Funding Statement
This work was supported by the Research Council of Kermanshah University of Medical Sciences (Grant Number: 96306).
Acknowledgements
The authors gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences (Grant Number: 96306) for the financial support.