Effects of Melatonin on Sleep Quality and Disease Activity in Patients With Rheumatoid Arthritis
Article information
Abstract
Background and Objective
In rheumatoid arthritis (RA) patients sleep disturbance is one of serious and prevalent problems. Considering the known effects of melatonin on sleep quality and inflammation, this study aimed to investigate melatonin supplementation effect on quality of sleep and disease activity in patients with RA.
Methods
In this randomized, placebo-controlled trial (double-blind), 64 RA patients were selected and divided into experimental and placebo groups randomly; experimental group received 3 mg/d of melatonin and another group consumed placebo for 60 days. Before and after the investigation, assessment of the quality of sleep determined using the Pittsburgh Sleep Quality Index (PSQI). Disease Activity Score-28 (DAS28) and the Visual Analogue Scale (VAS) questionnaires were used for evaluation of disease activity and pain intensity, respectively.
Results
Melatonin significantly reduced PSQI, DAS28 and VAS scores, when values compared with baseline. In contrast to placebo group, good sleep quality within the melatonin group increased significantly compared to baseline and this improvement in sleep quality was significant when compared between groups. The scores of DAS28 and pain VAS at the end of trial were significantly reduced compared to the baseline in both groups. However, reduction in the DAS28 and VAS scores of the melatonin group were stronger than reductions in the placebo receiving group.
Conclusions
This study results revealed that melatonin was safe and effective in improving sleep quality and reducing DAS28 and pain VAS scores in RA patients.
INTRODUCTION
Rheumatoid arthritis (RA), categorized as autoimmune originated disease, described by proliferation of synovial and higher inflammation status, detecting auto-antibodies specially rheumatoid factor as well as anti-citrullinated protein antibodies in serums, cartilage and bone erosion or malformation or simultaneous occurrence of all of above [1]. The prevalence of this disease in the world is about 1% and women are about 5 times more susceptible than men. The onset of the RA usually happens between the ages of 40–60 years in both genders. Its annual prevalence is reported to be about 460 per 100000 people [2]. Due to the fact that the symptoms of RA are manifested in the form of pain, swelling, fatigue, limited mobility, and joint fatigue, as a result, patients suffer from physical, mental, and social dysfunction and will generally have a deleterious impact on their quality of life [3]. Sleep disturbance is one of the most essential factors in reducing the quality of life in rheumatic disorders. Studies have shown that up to 70% of patients with RA suffer from sleep problems [4]. Sleep disturbance and poor sleep quality at night can worsen the symptoms of RA [5]. In particular, sleep problems and post-sleep fatigue are associated with low pain thresholds, increased pain intensity, depression, and inflammation in patients with RA [4]. Therefore, sleep quality is an important clinical issue in patients with RA and recognizing and solving sleep problems in these patients helps to improve their general health status.
Melatonin (methoxytryptamine-5-acetyl-N) is a neuronal hormone secreted by the pineal gland. Melatonin is produced during the night and regulates sleep, circadian rhythms, and seasonal changes in mammals’ behaviors [6]. Therefore, it can improve the quality of sleep and reduce fatigue during the day [7]. Studies have shown that exogenous melatonin can be beneficial in improving quality of sleep in cancer patients, chronic obstructive pulmonary disease, and multiple sclerosis [6]. However, few study has been performed in RA patients to convey the effect of exogenous melatonin on quality of sleep. Melatonin can also play an antioxidant and anti-inflammatory role. Therefore, due to the fact that RA is an inflammatory status and proinflammatory cytokines which are related to pathogenesis of RA for instance; interleukin-6 (IL-6), IL-1β, and tumor necrosis factor alpha (TNF-α) are increased, melatonin may be beneficial for reducing the rate of RA progression [8]. Studies show that higher levels of serum melatonin is effective against RA [9], while, some other studies showed that levels of melatonin in serum are already higher in RA patients [10]. Due to the fact that limited studies have been performed to investigate the beneficial effects of melatonin supplementation in RA patients, current study was aimed to survey the effect of melatonin supplementation on quality of sleep, pain and disease activity in RA.
METHODS
Study Design and Subjects
This “randomized placebo-controlled clinical trial (double-blind)” study was done in Ahvaz city in Iran. Samples were 18 to 50 years old that had body mass index (BMI) between 18.5 and 30 kg/m2 and known cases of RA. Exclusion criteria defined as: patients’ unwillingness to co-operate, use of antidepressants (such as sertraline and fluoxetine) or sedatives, smoking and alcohol consumption, use of any antioxidant supplements in the last 3 months, use of anticoagulants, pregnancy and breastfeeding, and presence of any disease other than RA.
According to consort flowchart of study illustrated in Fig. 1, 70 RA patients were participated in the study. The diagnosis of RA was according to rheumatologist clinical evaluation [11]. The selection of melatonin receiving group (n = 35) or placebo group (n = 35) was done using block randomization method.
The placebo or melatonin containing drugs were packaged in a visually same container by another research fellow to ensure that both patients and researchers remained completely blinded to the type of interventions. Patients were asked to take a pill containing 3 mg of melatonin or placebo one hour before bedtime for 60 days. Sublingual tablet form of melatonin (immediate releasing form, fast absorbing) was made by Vanadarogoster Co., Iran (packed with the name of Norm life Vanatonin®). The placebo contained cellulose and silicon dioxide. The placebo was made in the same color, shape, and size of melatonin supplement (Vanatonin®) by the Incubation Center for Pharmaceutical Technologies of Ahvaz Jundishapur University of Medical Sciences (AJUMS).
All participants were asked to report any possible side effects of the supplement during the study. Adherence was assessed by counting the remaining pills. Participants who took less than 90% of the prescribed pills were excluded from the study. All patients continued their usual medical treatment.
Ethics
The current randomized controlled trial (RCT) was approved and registered by the Medical Ethics Committee of AJUMS (No. IR.AJUMS.REC.1398.637). This RCT was also registered in the Iranian Registry of Clinical Trials (IRCT) (No. IRCT20191231 045964N1). Written informed consents were also taken from all participants.
Anthropometric Evaluation
Anthropometric measurements included height, weight, and BMI. Weight of participants was measured without shoes with light clothing via a digital scale (Seca, Hamburg, Germany; Model 831, accuracy = 100 g). Height (standing) was measured by using a wall measuring tape (accuracy = 0.5 cm, without shoes). BMI values were determined by dividing the weights by the squared values of heights (kg/m2).
Sleep Quality Assessment
The quality of sleep assessment was done by Pittsburgh Sleep Quality Index (PSQI) questionnaire. This tool assess the quality and disturbance of sleep in seven areas of: subjective quality of sleep, duration of sleep, sleep latency, efficiency of habitual sleep, disturbances of sleep, taking sleeping pills, and having dysfunction during daytime. Every item gets from zero to three points. The overall PSQI score is calculated by summation of scores from each of these seven areas in the range of zero to 21. Any PSQI score equal or more than five reflected poor quality of sleep and the higher score shows the worse quality of sleep [12]. This questionnaire validity and reliability in Iran have been investigated (correlation coefficient = 0.88 and α = 0.83) [13].
Assessing the Disease Activity (Severity of Rheumatoid Arthritis) and Visual Analogue Scale of Pain
Disease severity was calculated according to Disease Activity Score-28 (DAS28) and according to this formula:
DAS28 = 0.56 × √ (t28) + 0.28 × √ (sw28) + 0.70 × [Ln(ESR) + 0.014 × VAS]
where ESR is erythrocyte sedimentation rate.
This formula is an international formula for calculating the activity of RA. In this formula, “t” is the number of joints in pain and “sw” is the number of joints which are swollen and painful based on the Visual Analogue Scale (VAS).
Patients’ pain was measured as zero (no pain) to 100 (very high pain) according to the VAS. This criterion has been evaluated and calculated by a physician.
Statistical Analyses
Statistical analysis were done using SPSS software (V. 16, Chicago, IL, USA). Normality assumption was done using Kolmogorov–Smirnov test. Quantitative and categorical variables were represented as numbers (percentages) or means ± standard deviation, where applicable. Within groups comparisons were done using paired t-test. The between groups comparisons were done using chi-square test for qualitative or independent sample t-test for quantitative data. Moreover, the analysis of covariance (ANCOVA) using the multivariate general linear models was used for adjusting underlying factors which may confound the effectiveness of the intervention. p-values less than 0.05 were considered as statistically significant.
RESULTS
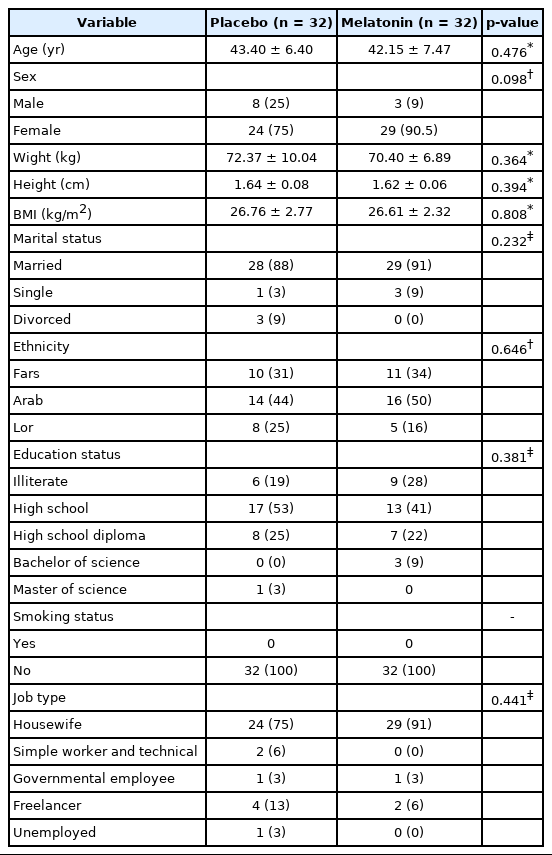
Out of 70 patients studied, 3 patients in both melatonin and placebo groups took less than 90% of the prescribed pills and 3 patients stated the unwillingness to continue, and were therefore excluded from the study (Fig. 1). Eventually, 64 participants completed the 60 days study (melatonin group, n = 32; placebo group, n = 32). The mean value for age of the subjects in the placebo and melatonin groups was 43.40 ± 6.40 and 42.15 ± 7.47 years old, respectively, without any significant difference. For BMI, the mean value in placebo and melatonin groups was 26.76 ± 2.77 and 26.61 ± 2.32 kg/m2, respectively, which no significant differences were seen (Table 1). Also, no significant difference found in the type of drugs consumption for RA therapy and other demographic characteristics when the two groups compared at the baseline (Table 2).
At the follow-ups, researchers ask for any kind of side effects caused after taking pills including headache, dizziness, gastrointestinal problems or nausea, drowsiness during the day, hypotension, and any kind of new symptoms during the intervention. Our follow-ups assessment revealed that no side effect was reported in the placebo group. However, two cases reported headache in the melatonin receiving group.
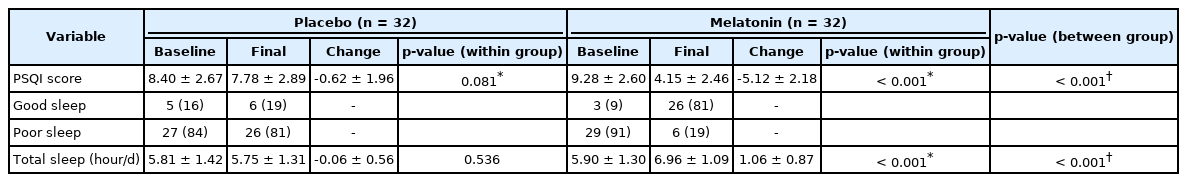
The mean PSQI scores of the two groups at the beginning and end of the interventions are shown in Table 3. After 60 days of trial, PSQI score within the melatonin group was significantly reduced, but no significant change found within the placebo group. Also, the changes in sleep quality between two groups across the study were statistically significant.
Good sleep (PSQI Score < 5) prevalence in patients within the placebo and melatonin groups at the beginning of the study were 16% and 9%, respectively. These outputs increased to 19% and 81% at the end of the study; indicating a dramatic increase in the melatonin group (Table 3). As well, the results of between groups’ differences tests showed that the changes in sleep quality were significantly different.
The mean score of DAS28 within two groups at the beginning and end of the intervention are shown in Table 4. The results from Table 4 revealed that the DAS28 values at the end of study in both of the melatonin and placebo receiving groups revealed a significant decrease when compared to the beginning of study. Despite of same trend in DAS28 levels during the study in both groups, the between groups differences revealed a statistically significant more reduction in disease activity level of the melatonin group.

Disease Activity and VAS scores assessments before and after interventions in rheumatoid arthritis patients
The mean VAS values for pain of the two groups at the beginning and end of the intervention are shown in Table 5. After 60 days the VAS value of pain decreased significantly in both melatonin and placebo groups, but the reduction in pain was significantly more in melatonin consumers.
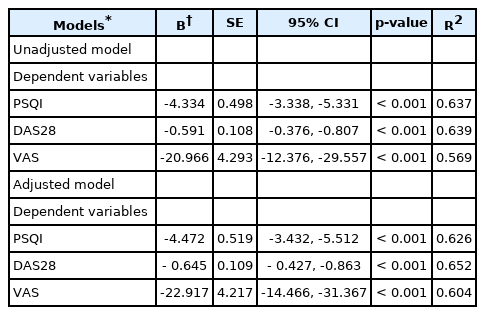
Comparing the effect of intervention (between groups) considering underlying factors by analysis of covariance
The influence of covariates such as age, sex, BMI, smoking, alcohol consumption, drug regimens, PSQI, VAS, and DAS28 on treatment effects (between groups) after ANCOVA are shown in Table 5.
DISCUSSION
We observed that 3 mg/day of melatonin supplementation in patients with RA for 60 days improved sleep quality, decreased DAS28 and VAS of pain scores.
Reports indicate that many patients with RA suffer from sleep disturbance, such as difficulty in falling asleep and/or early wake up, and increased daytime sleep [14]. Good sleep quality is important for regulating and repairing the body, while, sleep disturbance can worsen illness, daily disease activity, pain, morning stiffness, fatigue, or mood disorders [15]. In accordance to present study results, Son et al. [16] in their study showed that an increase in PSQI score in patients with RA was associated with an increase in DAS28 and VAS of pain scores, as some other studies have shown, too [17,18].
Melatonin is an endocrine neuronal hormone that can regulate the sleep-wake mechanism through a specific receptor mediator to play a sedative and sleep-inducing role. Melatonin supplementation has been used to improve sleep quality in various disease [19]. In some studies, the effectiveness of melatonin supplementation in sleep disturbance, blood pressure regulation, insomnia, metabolic syndrome, disorders related to oxidative damage, and neurological diseases has been reported [20-22]. Farshchian et al. [23] showed that using 3 mg/day of melatonin supplementation in cancer patients can significantly reduce PSQI score, which means enhanced sleep quality. Also, Shahrokhi et al. [24] in a study showed that 3 mg/day of melatonin supplementation in patients with colorectal cancer undergoing chemotherapy, significantly improves sleep quality. In a meta-analysis, Brzezinski et al. [25] evaluated results of 17 studies on effect of melatonin supplementation on sleep quality. Eventually their meta-analysis consists of 284 participants, revealed that exogenous melatonin could improve significantly some aspects of quality of sleep such as duration of sleep, efficiency of sleep, and sleep latency. Another meta-analysis consists of nine different studies included mental disabilities (a total of 183 participants). This study revealed that melatonin supplementation increased sleep time, reduced sleep latency, and decreased the number of participants awakenings per night [26]. Conversely, in another meta-analysis (included six RCT with 97 participants consist of all age groups), melatonin supplementation had no significant consequence on quality of sleep and sleep efficiency [27]. Moreover according to Buscemi et al. [27] study results, melatonin supplementation did not altered sleep latency.
Some studies suggested that the release of metalloproteinase and pro-inflammatory cytokines can be considered among the causes of RA which are related to environmental factors such as smoking, genetic (via epigenetics), microbiome culture, and also presence of periodontitis and can cause the destruction of cartilage and bone finally [28]. Some studies have shown that an increase in proinflammatory cytokines is directly related to the disease activity score in RA [8,29]. Some of the proinflammatory cytokines which facilitate the RA progression include IL-1β, TNF-α, and IL-6. Therefore, melatonin as an antioxidant and anti-inflammatory factor may be beneficial in the management of RA [30,31].
Hindering the signaling from nuclear factor-kappa β is one of the pathways that melatonin appears to protect cells from oxidative stress [31]. Therefore, with this justification, we can understand how melatonin defeats proinflammatory cytokines action such as IL-1β and TNF-α [32]. In addition, research shows that melatonin can dose-dependently suppress the proliferation of fibroblast-like synoviocytes (FLS, essential for RA pathogenesis) by activating the extracellular-signal-regulated kinase (ERK/P21/P27) pathway. These events imply that inhibition of FLS related damage in cartilage and bone of RA patients, could promise important consequences in the management of RA. Moreover, other pathways and inflammation-related molecules regulated by melatonin, such as; mitogen-activated protein kinase pathways associated with nuclear factor-erythroid-2–related factor 2 and also toll-like receptors (pivotal proteins in immune system) [33].
Another mechanism that can be introduced for the effect of melatonin on RA is the effect of melatonin on zinc-dependent matrix metalloproteinases (MMPs). MMPs are key enzymes involved in extracellular matrix (ECM) regeneration. MMPs play an important role in both physiological and pathological conditions. MMP-9 activity is associated with many pathological processes, including RA. As a result, inhibition of MMP-9 activity may be particularly effective in the control of RA diseases with unregulated ECM turnover. The results of a laboratory study showed that after binding of melatonin to its active site, it can inhibits the activity of the MMP 9, so it can be effective in reducing the severity of RA [34].
It should be noted that we have not considered a third group as the second control group without RA patients. Non-RA participants may have helped us to find out whether the beneficial effects of melatonin supplementation in RA patients are different from those in non-RA patients or not. Therefore, it seems that the known mechanism of action through MT1 and MT2 receptors (not specific to RA) may be involved.
On the other hand, contradictory results have also been reported that suggesting the ineffectiveness of melatonin supplementation in some of mentioned conditions. In a cross-sectional study in Iran, Afkhamizadeh et al. [9] assessed morning serum levels of melatonin in RA patients in order to compare with healthy individuals. They found no association between the serum levels of melatonin with DAS and even other factors including; disease duration, age, gender, medications, or sampling season in RA patients. Similarly, Esalatmanesh et al. [35] showed that 12 weeks of melatonin supplementation (6 mg/day) in patients with RA, although significantly reduced malondialdehyde (as a lipid peroxidation marker) and low-density lipoprotein cholesterol (LDL-C) levels in plasma, but had no effect on DAS. Therefore, it seems that different doses of melatonin in different treatment durations maybe is one of the reasons of different consequences. So, dose-dependent studies need to be performed at different duration of intervention in order to better understanding the effect of melatonin on RA.
Although no clinical trial about the antinociceptive effect of melatonin in RA was found in our search, several experimental studies demonstrated the melatonin analgesic effect in other pathologic conditions. There is some evidence of the analgesic effect of melatonin through membrane receptors (MT1/MT2) [36], nuclear receptors (RZRa/RZRb) [37], ion channels (activation of calcium channels) [38], and neurotransmitter systems (facilitation of GABAergic transmission) [39].
This study has some limitations. First, low sample size. Other limitations were that we did not measure serum oxidative stress and inflammatory markers, and metabolic panels because of financial constraints. In addition, lack of serum melatonin levels assessment led to hindering patients who had less compliance in supplement consumption. Besides, the difference in melatonin baseline levels among participants may be considered as a confounder. While different foods may contain some amounts of melatonin, managing patients’ dietary intakes during the study seems to be necessary. Finally, a third group comprising non-RA participants (as a second control group) would help us to prove the different mechanisms of action for melatonin in RA.
In conclusion, melatonin supplementation can improve sleep quality and reduce DAS28 and VAS of pain scores in RA patients. Nevertheless, more researches are needed with larger sample sizes as well as considering other factors, including plasma levels of melatonin and inflammatory markers, to better comprehend the effects of melatonin on RA.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Authors’ Contributions
Conceptualization: all authors. Data curation: all authors. Formal analysis: Tayebeh Palimi, Marzie Zilaee. Funding acquisition: Elham Rajaei, Majid Karandish. Investigation: all authors. Methodology: Marzie Zilaee, Elham Rajaei, Majid Karandish. Project administration: all authors. Resources: all authors. Software: all authors. Supervision: Elham Rajaei, Majid Karandish. Validation: all authors. Visualization: all authors. Writing—original draft: all authors. Writing—review & editing: all authors.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Funding Statement
This study was financially supported by the Vice-chancellor for Research Affairs of Ahvaz Jundishapur University of Medical Sciences (grant No. NRC9819).
Acknowledgements
This study was extracted from Tayebeh Palimi’s MSc thesis in Nutrition.