Relationship of Subjective and Objective Sleep Quality with Caregiver Burden in Patients with Alzheimer’s Disease
Article information
Abstract
Background and Objective
Sleep disturbance in patients with Alzheimer’s disease (AD) could increase institutionalization due to caregiver burden. The objective of this study was to examine the relationship of subjective and objective sleep quality with caregiver burden in AD patients with insomnia symptoms.
Methods
AD patients of mild to moderate degree were recruited. They were assessed with the Pittsburgh Sleep Quality Index (PSQI), Korean version of the Epworth Sleepiness Scale, and Mini-Mental Status Examination in the Korean version of Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet. Caregiver burden was evaluated with Korean version of the Neuropsychiatric Inventory Questionnaire (KNPI-Q) and Korean version of the Zarit Burden Interview (ZBI-K). Actigraphy recording (Actiwatch 2; Philips Respironics) was conducted for five days at home. Thirty-four patients (age 78.44 ± 6.14, M:F = 9:25) were analyzed. These patients were divided into two groups by the median of KNPI-Q for caregiver distress [KNPI-Q(d)] and ZBI-K scores. Sleep parameters were compared between the two groups.
Results
Among seven components of PSQI, sleep latency was correlated with KNPI-Q(d) score. The patients with higher KNPI-Q(d) scores had lower sleep efficiency (SE) (p = 0.002), greater wake after sleep onset (p = 0.037), and higher fragmentation index (p = 0.040) than those with lower KNPI-Q(d) scores. Those with higher ZBI-K scores had lower SE (p = 0.026) than those with lower ZBI-K scores. Stepwise multiple regression revealed that SE predicted 16% of KNPI-Q(d) scores (p = 0.012).
Conclusions
Difficulty in sleep initiation according to subjective reports was associated with caregiver burden in our AD patients. In objective sleep quality, a significant difference in difficulty of sleep maintenance was found between higher and lower caregiver burden groups. This suggests that sleep maintenance is essential for reducing caregiver burden.
INTRODUCTION
Dementia is an illness with a cognitive decline that causes social and/or occupational impairments. The Ministry of Health and Welfare of the Republic of Korea reported that the prevalence of dementia in South Korea had reached 10.16% among the elderly aged over 65 years in 2018 [1]. The most common cause of dementia is Alzheimer’s disease (AD) [1]. It has been a long while since sleep disturbance in the elderly population has been implicated as one of AD etiologies. A prospective study using actigraph in the elderly without dementia has shown that sleep fragmentation due to awakenings during the night can increase the AD risk by 1.5 times after six years of follow-up [2]. Furthermore, sleep disturbance as a modifiable risk factor of AD is clinically important [3].
Behavioral psychological symptoms of dementia (BPSD) can lead to early institutionalization of patients, poor quality of life of caregivers, and increased medical expenses [4]. In a study that identified factors affecting the caregiver burden of AD patients, BPSD including hallucinations, abnormal behaviors, and insomnia was the most significant in AD patients [5]. It also found that the severity of BPSD rather than that of cognitive decline was correlated with increased caregiver burden [5]. In particular, the presence of insomnia of BPSD in AD patients can highly contribute to increased caregiver burden. In a previous study, caregiver burden has been found to be significantly associated with sleep disturbance symptoms, including nocturnal awakenings, nocturnal wandering, snoring, and daytime sleepiness, in AD patients [6]. In addition, insomnia itself in the early stage of cognitive decline may predict the progression of AD [7], implicating that insomnia should not be just regarded as one of BPSD.
Insomnia in AD is very common. It occurs even at its early stage [8]. It is related to neurodegeneration of the suprachiasmatic nucleus regulating the sleep wake rhythm [9]. Besides, AD patients may feel nervous, restless, and anxious, especially in the early evening, indicating that sundowning syndrome or nocturnal delirium is closely associated with their disrupted circadian rhythm that can greatly affect the quality of life of both patients and their caregivers [10]. Furthermore, it has been suggested that high caregiver burden could negatively impact the course of AD [11].
Incidentally, a previous study has suggested that there is a discrepancy between subjective and objective sleep in early and moderate stage of AD, showing that AD patients complain less of insomnia, while their objective sleep parameters indicate more disturbed sleep, including a longer sleep onset latency (SOL) and a lower sleep efficiency (SE) [12]. Although polysomnography is known as a gold standard for assessing the objective sleep, its reliability and cost effectiveness are low in patients with dementia due to a large ‘first night effect’ or compliance problems. On the other hand, actigraph as another tool for assessing objective sleep is more favored for AD patients since objective sleep can be assessed for multiple days at a home-based setting [13].
Neuropsychiatric Inventory (NPI) [14], Behavioral Pathology in Alzheimer’s Disease Rating Scale [15], and Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [16] are tools for evaluating BPSD in patients with dementia. The validity and reliability of the Korean version of NPI [17] for evaluating the severity of 12 typical BPSD and the degree of suffering of caregivers have been verified [18]. Zarit Burden Interview (ZBI) [19] and Caregiver Burden Inventory [20] can be used for assessing caregiver burden. ZBI shows high consistency and reliability. Thus, it usually used to measure caregiver burden. The objective of this study was to examine the relationship of subjective sleep quality and objective sleep parameters in AD patients with caregiver burden, comparing sleep parameters between the two groups divided by median values of the Korean version of the NPI Questionnaire (KNPI-Q) and the Korean version of ZBI [21] score. In addition, factors affecting caregiver burden among objective sleep parameters were determined. Our hypothesis was that subjective and objective poor sleep quality in AD patients would be associated with their caregiver burden.
METHODS
Subjects
Patients with AD-dementia who visited the Dementia Clinic at Kangwon National University Hospital and their caregivers from March 2017 to February 2019 were enrolled in this study. Participants in this study had been recruited for the purpose of participating in an intervention study to investigate the effectiveness of timed blue-enriched light treatment in patients aged 50 or more with mild and moderate AD who had a sleep disturbance. The diagnosis of AD-dementia and determination of its severity were performed using the Diagnostic and Statistical Manual of Mental Disorder, 5th edition (DSM-5) [22] and the Clinical Dementia Rating Scale (CDR) [23], respectively. Inclusion criteria were a diagnosis of major neurocognitive disorder (NCD) due to either probable or possible AD and a CDR score of 0.5 to 2. A sleep disturbance was verified by a score of 5 or greater for the Pittsburgh Sleep Quality Index (PSQI) [24] and/or by one or more complaints among difficulty initiating sleep, difficulty maintaining sleep, and early morning awakening for three or more days per week. Patients were excluded if they met the following criteria: 1) current substance related disorders, depressive disorders, or other psychiatric disorders by DSM-5; 2) current medical illness including liver cirrhosis, chronic pulmonary disease, cancer, uncontrolled diabetes, and uncontrolled hypertension; 3) being suspected or diagnosed with primary sleep disorders except for insomnia disorder (i.e., restless legs syndrome, sleep-disordered breathing disorder; hypersomnia, or narcolepsy); 4) a prior history of cerebrovascular disease or central nervous system (CNS) disease, or evidence of CNS injury.
The study protocol was approved by the Institutional Review Board of Kangwon National University Hospital (B-2016-10-007). Written informed consent was obtained from each participant and his/her legally authorized representatives prior to commencement of this study. All procedures were carried out in accordance with principles of the Declaration of Helsinki.
Measures
The diagnosis of major NCD due to AD and determination of its severity through clinical interviews were performed by a psychiatrist or a neurologist. The neuropsychological evaluation was based on the Korean version of Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-K) Neuropsychological Assessment Battery. Other medical conditions and the use of medications or other substances that might affect their behavior and cognitive function were also examined.
For eligible patients, self-reported questionnaires including Activity of Daily Living (ADL), the Korean version of Geriatric Depression Scale (GDS-K), and the Korean version of Epworth Sleepiness Scale (KESS) were administered. Patients with KESS score ≥ 12 were further evaluated to determine whether they had any primary sleep disorder except insomnia disorder by a psychiatrist through a clinical interview.
Behavior symptoms of AD patients were assessed using the Korean version of the Neuropsychiatric Inventory Questionnaire (severity) [KNPI-Q(s)]. The Korean version of the Zarit Burden Interview (ZBI-K) and the KNPI-Q for caregiver distress [KNPI-Q(d)] were used for assessing caregiver burden. Actigraphy recording (Actiwatch 2; Philips Respironics, Murrysville, PA, USA) and sleep diary were obtained for five consecutive days at home.
Thirty-nine AD patients were enrolled, but the five among them were excluded due to refusal or no documentation of sleep diary. Then, thirty-four AD patients (age: 78.44 ± 6.14 years; 9 males; education of 5.47 ± 3.89 years) and their caregivers (age: 76.1 ± 5.9 years) were included in the final analysis of our study.
Korean version of the Neuropsychiatric Inventory Questionnaire (KNPI-Q) [17]
As a brief questionnaire form of the NPI, the KNPI-Q evaluates 12 neuropsychiatric symptoms (NPS), including delusion, hallucinations, agitation/aggression, depression/dysphoria, anxiety, euphoria/elation, apathy/indifference, disinhibition, irritability/lability, aberrant motor, night-time behavior, appetite/eating. The KNPI-Q provides NPS severity of demented patients [KNPI-Q(s), 3-point scale, a total of 36 points] and distress ratings of their caregiver for each symptom reported [KNPI-Q(d), 5-point scale, a total of 60 points].
Korean version of Zarit Burden Interview (ZBI-K) [21]
The ZBI was originally developed to assess burden of caregivers for community-dwelling persons with dementia. It evaluates the perceived impact of caregiving on aspects such as caregiver’s health, personal and social life, financial situation, emotional wellbeing, and interpersonal relationships. It consists of 22 items rated on a 5-point Likert scale from 0 (never) to 4 (nearly always). Its total score ranged from 0 to 88, with a higher score indicating a higher caregiver burden.
Actigraphy
Actiwatch recording was performed at one-min epochs using a wake-threshold value of 40 activity counts. Actigraphy data were derived with an Actiware-Sleep software version 6.0.2 (Philips Respironics). Invalid actigraphy data were excluded due to problems such as technical errors of Actiwatch software and fairly deviated sleep-wake patterns caused by special events or alcohol drinking. Data without movement and/or light signals for one hour or more were treated as missing data. When compliance with wearing the Actiwatch was problematic (i.e., no movement or light signals for 4 h/day or more), data for those days were discarded. Objective nocturnal sleep parameters were calculated. Sleep parameters included the following: time in bed, total sleep time (TST), sleep onset, SOL, wake time after sleep onset (WASO), SE, and fragmentation index (FI).
Statistical analysis
Descriptive statistics [mean, standard deviation (SD) and range] were done for the variables of demographic and clinical data, the scores of GDS-K, Mini-Mental Status Examination in the Korean version of CERAD Packet (MMSE-KC), KESS, PSQI, KNPI-Q, and ZBI-K, and objective sleep parameters in total patients. A partial correlation analysis controlling for age, sex, education level, GDS-K, and CDR was conducted to evaluate relationships of the degree of caregiver burden with the scores of MMSE-KC and KNPI-Q(s), the scores of PSQI global and its seven components, and sleep parameters in total patients.
Patients were divided into two groups by the median of KNPI-Q(d) scores. The MMSE-KC scores, the scores of PSQI global and its seven components, and objective sleep parameters were compared between the group with higher KNPI-Q(d) scores and the group with lower KNPI-Q(d) scores using the Mann-Whitney U test. These variables were also compared between the group with higher ZBI-K scores and the group with lower ZBI-K scores distinguished by the median of ZBI-K scores. A stepwise multiple regression analysis was used to identify independent objective sleep parameters [bed time (BT), SE, WASO, and FI] predicting the KNPI-Q(d) score in total patients. All statistical analyses were performed using SPSS software package, version 18.0 (SPSS Inc., Chicago, IL, USA). Two-sided p values of less than 0.05 were considered statistically significant.
RESULTS
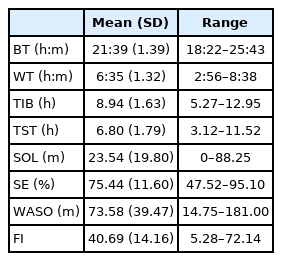
Demographic and clinical characteristics of AD patients are shown in Table 1. Their mean ADL score (SD) was 4.28 (1.94). CDR scores were 0.5, 1, and 2 for 15, 13, and 6 patients, respectively. Their mean GDS-K, MMSE-KC, KNPI-Q(s), and KNPI-Q(d) scores (SD) were 11.37 (7.47), 16.09 (4.48), 9.32 (6.37), and 10.38 (9.21), respectively. Their mean KESS, PSQI, and ZBI-K scores (SD) were 4.82 (5.46), 10.41 (4.27), and 34.35 (16.14), respectively.

Demographic and clinical characteristics and caregiver burden in the patients with Alzheimer’s disease (n = 34)
Results of objective sleep parameters are shown in Table 2. Mean SOL (SD) of patients was 23.54 ± 19.80 min. Their SE, WASO, and FI were 75.44 ± 11.60%, 73.58 ± 39.47 min, and 40.69 ± 14.16, respectively.
There was no significant correlation between scores of MMSE-KC and scores of KNPI-Q(d) or ZBI-K of our AD patients. However, their KNPI-Q(s) scores were significantly correlated with ZBI-K scores (r = 0.684, p < 0.001). PSQI global scores were not significantly correlated with scores of KNPI-Q(d) or ZBI-K, whereas scores of sleep latency among seven components of PSQI were correlated with ZBI-K scores (r = 0.396, p = 0.045). On the other hand, objective sleep parameters had no significant correlation with scores of KNPI-Q(d) or ZBI-K (Table 3).
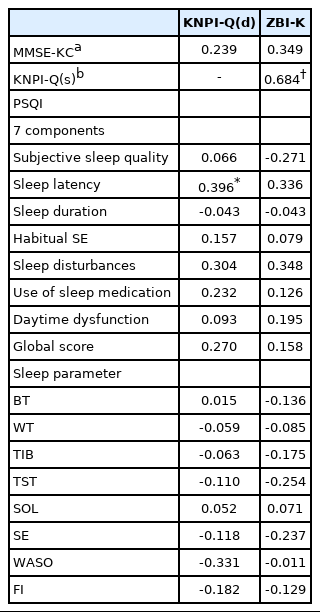
Correlations of the scores of MMSE-KC, KNPI-Q(s), PSQI, and sleep parameters with the caregiver burden in the patients with Alzheimer’s disease (n = 34)
There was no significant difference in the MMSE-KC score, PSQI global score, or score of seven components of PSQI between the higher and lower KNPI-Q(d) groups, while the higher KNPI-Q(d) group had a significantly earlier BT, a lower SE, a greater WASO, and a higher FI than the lower KNPI-Q(d) group (p < 0.05 or p < 0.01) (Table 4).

Comparisons of the scores of MMSE-KC, PSQI, and sleep parameters between the AD patients with lower KNPI-Q(d) scores and those with higher KNPI-Q(d) scores
There were no significant differences in MMSE-KC scores, scores of PSQI global, or scores of seven components of PSQI between higher and lower ZBI-K groups. However, the higher ZBI-K group had significantly higher KNPI-Q(s) scores (p < 0.01) and lower SE (p < 0.05) than the lower ZBI-K group (Table 5).
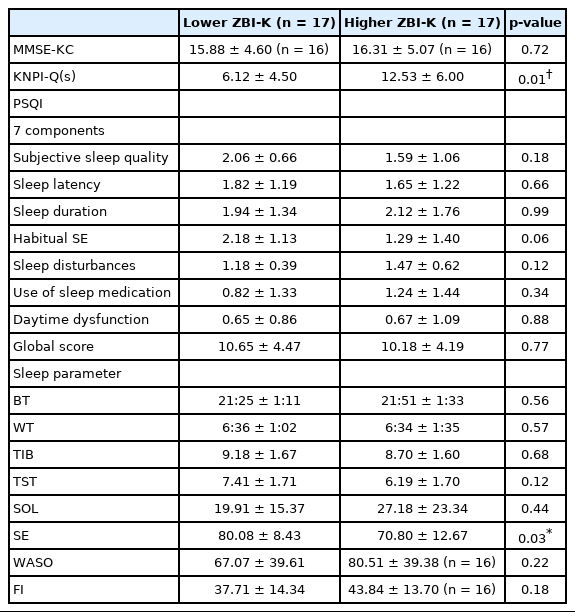
Comparisons of the scores of MMSE-KC, KNPI-Q(s), PSQI, and sleep parameters between the AD patients with lower ZBI-K scores and those with higher ZBI-K scores
Stepwise multiple regression models revealed that among BT, SE, WASO, and FI, only SE was a significant predictor of the KNPI-Q(d) score. It explained 16% of the variance in the KNPI-Q(d) score (Table 6).
DISCUSSION
In our study, the mean age of patients with AD-dementia was 78.44 years. There were 9 males and 25 females, showing a relatively high ratio of females. Their mean education level was 5.47 years, reflecting that many of them had elementary school education, similar to findings of a previous epidemiological study [25]. Fifteen, thirteen, and six of our AD patients had CDR scores of 0.5, 1, and 2, respectively. The mean MMSE-KC score of these AD patients was 16.09. In our AD patients, the mean GDS-K score was 11.37. Although six patients had GDS-K scores of 20 or more, they did not meet the criteria of major depressive disorder. The mean KESS score of our AD patients was 4.82. Five patients had KESS scores of 10 or more. However, they were not suspected clinically of having primary sleep disorders. The mean PSQI total score of AD patients was 10.41, indicating that their subjective sleep quality was relatively poor (Table 1). In general, insomnia as well as cognitive decline is associated with advancing age. High prevalence of insomnia in the elderly could be considered as a consequence of changes in sleep architecture [26]. Shin et al. [27] have reported that the mean PSQI score of AD patients (mean age of 74.83 years, n = 63) is 5.44. Grace et al. [28] have shown that the mean PSQI score of AD patients (mean age of 74 years, n = 20) is 4.6.
In our study, the SOL, SE, and WASO of our AD patients were 23.54 minutes, 75.44%, and 73.58 minutes, respectively, indicating that their objective sleep quality was poor (Table 2). A previous study has measured sleep parameters using actigraph in AD patients and found that they have SOL of 14.2 minutes and WASO of 70.3 minutes [29]. Leger et al. [30] have used actigraph and found that mild and moderate AD patients have WASO of 58 minutes and 61 minutes, respectively. Our patients might have worse subjective and objective sleep quality than AD patients reported in these previous studies.
The purpose of our study was to determine whether cognitive decline, BPSD, and subjective and objective sleep quality might be associated with caregiver burden assessed by the KNPI-Q(d) and ZBI-K. Although the cognitive decline assessed by the MMSE-KC was not associated with caregiver burden, the severity of BPSD assessed by the KNPI-Q(s) was significantly associated with caregiver burden assessed by the ZBI-K (r = 0.684, p < 0.01) (Table 3). Allegri et al. [5] have shown that caregiver burden is not related to MMSE or CDR score, although it is related to the NPI score. Lou et al. [31] have also reported that caregiver burden is not related to MMSE scores, although it is related to the NPI score. In addition, a meta-analysis has indicated that factors associated with caregiver’s quality of life cannot be predicted by the degree of cognitive and functional decline in dementia patients, but by the degree of their BPSD [32].
In our study, there was no association of caregiver burden with total score of the PSQI, although there was a positive correlation of caregiver burden with the score of sleep latency, one of seven components of PSQI (r = 0.396, p < 0.05) (Table 3). Okuda et al. [33] have reported that self-reported poor sleep quality of AD patients assessed by the Sleep Disorders Inventory (SDI) is associated with increased caregiver burden. The SDI is a questionnaire that evaluates the severity and frequency of sleep-related problems derived from the NPI (insomnia, night roaming, daytime sleep, etc.). Zhou et al. [34] have reported that higher PSQI total scores and scores of sleep latency from PSQI components in AD patients are associated with higher NPI total scores. Meanwhile, Cupidi et al. [35] have analyzed scores of the seven components of the PSQI for caregivers of AD patients and found that their PSQI total scores and scores for two components (sleep latency and sleep disturbances) are significantly increased compared to those scores of controls. There is a possibility that their caregivers could not sleep until AD patients fall asleep, leading to increased caregiver burden. Although many studies have examined subjective sleep quality of AD patients [36] using the PSQI, studies that investigate the association between each PSQI component score and caregiver burden are lacking.
In our study, sleep parameters measured by actigraph were not associated with caregiver burden. We then determined differences in sleep parameters between the two groups divided by median values of KNPI-Q(d) and ZBI-K. However, we found no significant difference in global cognitive functioning measured by the MMSE-KC between the two groups. There was no significant difference in subjective sleep quality assessed by total score or component scores of the PSQI between the two groups either. Among objective sleep parameters measured by actigraph, the group with higher KNPI-Q(d) score had significantly lower SE (p = 0.002), greater WASO (p = 0.037), and higher FI (p = 0.040) than the group with lower KNPI-Q(d) score (Table 4). One of domains of KNPI-Q encompasses ‘night-time behavior,’ which might reflect the degree of sleep disturbance in our AD patients. However, there was no significant difference in the mean score of night-time behavior between higher and lower KNPI-Q(d) groups (2.4 ± 0.7 vs. 1.4 ± 1.1, p = 0.08). Thus, the group difference of objective sleep quality is unlikely to be linked to the difference in the severity of night time behavior.
In our study, AD patients with higher KNPI-Q(d) scores had earlier bedtime, lower SE and higher FI than those with lower KNPI-Q(d) scores. It is more likely that a difficulty in sleep maintenance rather than sleep initiation is associated with higher caregiver burden. An increase in the score of sleep latency as one of PSQI components was associated with increased caregiver burden assessed by KNPI-Q(d), while the SOL of objective sleep parameters did not show a significant difference between AD patients with higher and lower KNPI-Q(d) scores (Tables 4 and 5). Our finding was meaningful in that we assessed the sleep quality of AD patients based on objective measures as well as subjective reports. Interestingly, our findings showed that objective sleep parameters indicating sleep maintenance problem were associated with increased caregiver burden.
When we divided our AD patients into two groups according to the median of ZBI-K scores, there was no significant difference in cognitive function measured by the MMSE-KC between the two groups. Meanwhile, the severity of BPSD measured by KNPI-Q(s) showed a significant (p < 0.001) difference between the two groups (Table 5). Our findings are consistent with result of previous study [37] showing that BPSDs are related to caregiver’s poor quality of life, patient’s early institutionalization, and increased medical expenses. Regarding subjective sleep quality, there was no significant difference in total score of PSQI or component score of the PSQI between AD patients with lower ZBI-K scores and those with higher ZBI-K scores. Among objective sleep parameters, the SE measured by actigraph was significantly lower in the group of higher ZBI-K scores than the group of lower ZBI-K scores (p = 0.03), consistent with the finding showing that the group of higher KNPI-Q(d) scores had a significantly lower SE than the group of lower KNPI-Q(d) scores (Table 4). There were limited studies on the relationship between objective sleep quality of AD patients and their caregiver burden. A previous study using actigraph has reported that older nursing-home residents with dementia have significant lower SE (77.4%) and longer awake time (103.6 min) throughout the night than those without dementia, indicating a worse sleep quality, particularly concerning difficulty of sleep maintenance in dementia patients [38]. However, it would be difficult to simply compare our finding with result of that previous study which was not focused on AD patients only.
Insomnia in AD patients seems to be associated with higher caregiver burden which negatively affects the sleep of caregivers. Peng et al. [39] have reported that sleep problems of dementia patients can predict decreases of TST and SE measured by actigraph in addition to subjective poor sleep quality and daytime dysfunction of their caregivers. The present study also revealed that their insomnia was associated with increased depression, burn out, and care burden of caregivers for dementia patients. In our AD patients, SE, WASO, BT, and FI of objective sleep parameters were associated with high caregiver burden. A stepwise multiple regression analysis revealed that among variables associated with sleep, the SE predicted 16% of KNPI-Q(d) scores (Table 6). This finding might indicate that the low SE of AD patients can lead to insomnia of their caregivers, thus increasing the caregiver burden. Ryuno et al. [40] have reported that increased caregiver burden is associated with higher PSQI score (r = 0.62) and less TST measured by actigraph (r = -0.42) of caregivers.
Our study has some limitations. First, we only included patients with mild or moderate degree of AD. Second, our AD patients had a sleep disturbance. Thus, it would be difficult to generalize our results to all AD patients. Third, it was difficult to completely exclude patients with a primary sleep disorder because its presence in our patients was assessed only by clinical interview without conducting polysomnography. Fourth, various covariates including the use of medications and the comorbidity of medical conditions were not adjusted for, although these confounding factors could influence our outcomes. Lastly, another neurodegenerative or cerebrovascular disease other than AD might have contributed to the dementia in our patients at the same time since genetic mutation analysis and neuroimaging were not performed.
In conclusion, regarding subjective sleep quality, scores of sleep latency from PSQI component were associated with increased KNPI-Q(d) scores, indicating a relationship of difficulty in sleep initiation with increased caregiver burden. However, regarding objective sleep quality, the group with higher caregiver burden showed a significantly lower SE and a greater WASO than the group with lower caregiver burden. This indicates that those with higher caregiver burden have more difficulty in sleep maintenance.
Our study implicates the possibility that both difficulties of sleep initiation and sleep maintenance in AD patients can lead to increased caregiver burden. Our study is particularly meaningful in that the difficulty of sleep maintenance objectively measured for AD patients is related to caregiver burden. Considering that studies on the association of caregiver burden with sleep quality measured both subjectively and objectively in AD patients are limited, further studies are needed.
Acknowledgments
This study was supported by a grant (2017R1A2B4003493) of the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning, Republic of Korea. It was also supported by a 2016 Research Grant (No. 520160249) from Kangwon National University. We thank participants and their caregivers in Gangwon-do, Republic of Korea.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Authors’ Contribution
Conceptualization: Lee JH. Data curation: Lee JH, Kim SJ. Formal analysis: Lee EJ, Kim SJ. Funding acquisition: Lee JH. Investigation: Lee SH, Lee JH, Kim SJ, Jang JW, Jhoo JH. Methodology: Kim SJ, Lee JH. Project administration: Lee JH. Resources: Jang JW, Jhoo JH. Supervision: Lee JH. Writing—original draft: Lee EJ. Writing—review & editing: Kim SJ, Lee JH.