Drug-Induced Sleep Endoscopy: A Guide for Treatment Selection
Article information
Abstract
Identifying the sites, severity, and pattern of upper airway obstruction in obstructive sleep apnea is crucial for determining the treatment options, particularly the surgical plan. Although early methods of assessment, such as Muller’s maneuver, computed tomography, and magnetic resonance imaging in awake patients have been utilized to assess obstructive sites, the physiologic and anatomical differences between awake and sleeping patients showed a limited ability of those methods as diagnostic tools. Recently, drug-induced sleep endoscopy (DISE) was introduced as a useful diagnostic and evaluation tool to identify dynamic upper airway collapse during sedation that simulates natural sleep. In this article, we review all aspects of DISE, including the technique, evaluation methods, and clinical application.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep disorder, characterized by recurrent collapse of the upper airways during sleep. Because a wide spectrum of pharyngeal dysfunction is found in OSA, evaluation of the obstruction site and its severity in patients with OSA is extremely important for selecting the treatment method. There are four treatment options for OSA, including positive airway pressure (PAP), oral devices, surgery, and lifestyle modifications. To date, PAP is thought to be the most effective treatment but shows the problem of compliance. Therefore, surgery and oral devices play specific roles for some patients with OSA. Although the expected success rate of surgery is relatively lower than that of PAP, it is an effective option for patients who have obvious anatomic problems or want to be treated with surgery at once. In those patients, it is mandatory to evaluate the obstructive level of the airways during sleep. Several techniques for evaluation of the airways have been proposed, including clinical examination with Muller’s maneuver, cephalometry, computed tomography (CT), magnetic resonance imaging (MRI), acoustic reflectometry, sleep videofluoroscopy (SVF), and drug-induced sleep endoscopy (DISE). Although early methods of evaluation, such as Muller’s maneuver, CT, and MRI in awake patients were utilized to assess the obstructive site, the physiologic and anatomical differences between awake and sleeping patients showed a limited ability as evaluation tools. SVF and DISE can be used under sedation mimicking sleep. SVF has several advantages: dynamic sleep study, performed in the supine position, and visualization of the both the head and neck, including bony and soft structures and upper airways together. However, it has the limitations of radiation exposure, superimposition of structures, and two-dimensional view. Recently, most OSA surgeons utilized DISE to determine the surgical options, particularly using general anesthesia, although DISE has disadvantages related to identifying the entire airways together.
As observation of the obstructive site during sleep is necessary for proper evaluation, Croft and Pringle [1] first suggested “sleep nasoendoscopy” to assess OSA patients in 1991. As a pharmacological agent is required to sedate patients, Kerzirian and Hohenhorst [2] changed the nomenclature to “drug-induced sleep endoscopy.” In contrast to the previous procedures that commonly provide two-dimensional evaluations in the upright sitting and awake state, DISE provides a three-dimensional assessment of the upper airways during unconscious sedation that simulates natural sleep.
TECHNIQUE
Preparation and Patient Positioning
Eating should be restricted for patients before the procedure to prevent aspiration during the procedure. Anticholinergic agents, such as atropine and glycopyrrolate, can be administrated 30 min before the procedure to decrease salivary secretion, which leads to a better window of the upper airway and reduces coughing caused by aspiration. A local decongestant was commonly applied to the unilateral or bilateral nasal airways. The amount of local anesthesia should be minimalized to avoid anesthetic extension to the pharynx and affecting the genioglossus [3]. Endoscopic examination should be performed before administration of the sedative to verify the anesthetic state.
Sedative Agents
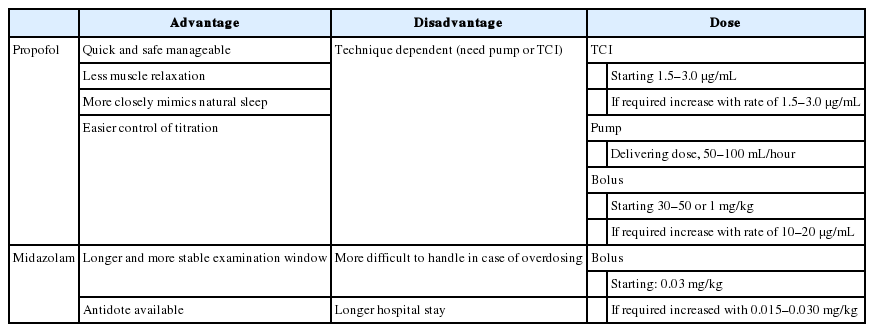
Propofol (2-6-diisopropylphenol) and midazolam (benzodiazepine) are commonly used as sedative agents during DISE. Dexmedetomidine (alpha-2 adrenergic receptor agonist) is also utilized. Propofol is a hypnotic agent, but its mechanism of its action remains unknown; it is believed that gamma-amino-butyric acid (GABA) circuits are associated with the mechanism of action of propofol. It can reproduce slow waves on electroencephalogram, which resembles the waves of non-rapid eye movement (NREM) sleep. Previous studies demonstrated that pharyngeal pressures under propofol sedation were equal to those in natural NREM sleep [4,5]. Midazolam is a short-acting benzodiazepine that increases the opening frequency of the GABA-A receptor. Theoretically, it produces greater relaxation of the pharyngeal muscle compared to propofol. However, studies measuring the pharyngeal pressure showed that pharyngeal pressures under midazolam sedation also corresponded to those of NREM natural sleep [6,7]. Table 1 describes the advantages, disadvantages, and doses of propofol and midazolam.
Setting and Monitoring
DISE is safely performed in an operating room or a procedure suite but can also be performed in an outpatient setting with the basic resuscitation equipment (e.g., supplemental oxygen) and available personnel for safe administration of the sedative. Comfortable room temperature, a quiet environment, and dim lights are helpful to simulate natural sleep. The oxygen saturation, heart rate, and blood pressure must be monitored during the procedure, and supplemental oxygen should be prepared in case of emergency. Sedation depth can be assessed with the loss of consciousness, onset of snoring, and disordered breathing events. The bispectral index score (BIS) provides a quantified sedation depth. The recommended target BIS range during DISE is 50 to 70 [8]. A previous study demonstrated more upper airway collapse in deep sedation (BIS of 50 to 60) compared to light sedation (BIS of 65–75), which suggested that a higher level of sedation may cause a greater loss of muscle tone [9]. Therefore, a constant sedation depth should be set with a definite protocol for the individual sleep laboratory.
Evaluation of the Upper Airways in DISE
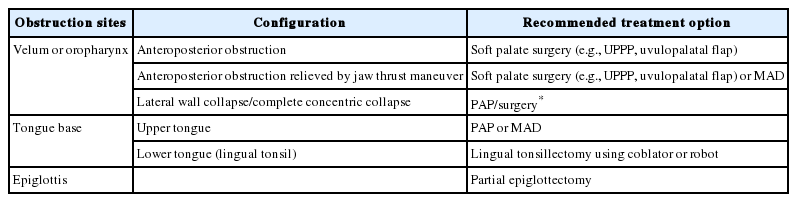
Various classifications have been reported to describe DISE findings. Although there is no consensus, the VOTE classification is the most widely used because it integrates the four major structures that contribute to upper airway obstruction in most patients: velum, oropharyngeal lateral walls, tongue base, and epiglottis [10]. Fig. 1 shows the four sites of obstruction in the VOTE classification. In addition, the NOHL classification, which includes the nose, oropharynx, hypopharynx, and larynx, is used [11]. Table 2 summarizes these two classification systems. VOTE and NOHL classifications share similarities in most parameters to identify the site, degree, and pattern of obstruction of the upper airways. The NOHL classification may be more advantageous because it provides an additional level of nasal obstruction. In contrast, a previous study comparing VOTE and NOHL classification showed that the VOTE classification was more comprehensive in the assessment of the pharynx and epiglottis [12].
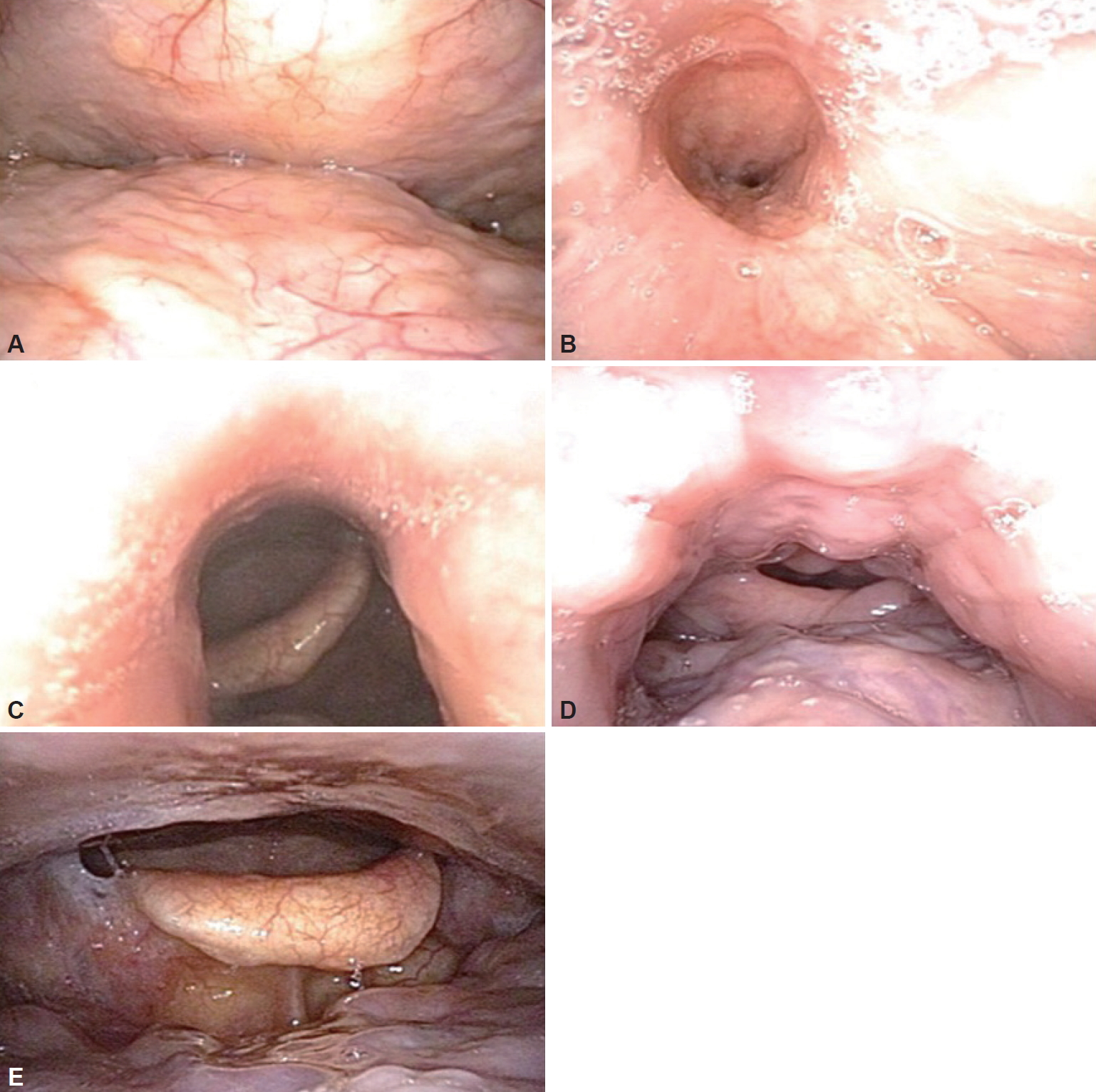
VOTE classification. A: Velum obstruction in the anteroposterior configuration. B: Velum obstruction in the concentric configuration. C: Oropharyngeal lateral wall collapse. D: Tongue-related obstruction. E: Epiglottic collapse in the anteroposterior configuration. VOTE: velum, oropharyngeal lateral walls, tongue base, and epiglottis.
CLINICAL APPLICATION
The clinical purpose of DISE is to guide treatment selection based on the association between the findings in DISE and outcomes of various treatment modalities. Multiple studies have examined the DISE as a diagnostic tool that aids in treatment planning [13-16]. A study described that DISE may change the treatment recommendations in 78% of subjects [15]. Since it does not evaluate the severity of OSA, complete polysomnography or portable monitoring should be performed before DISE. Some aspects of the upper airway anatomy are better observed in the awake state; therefore, a complete awake upper airway examination is also important to aid in treatment selection. DISE is indicated for OSA patients and simple snorers when additional information provided by DISE is considered important. For example, DISE is not necessary for all patients treated with continuous positive airway pressure (CPAP). However, if the patient does not tolerate CPAP, DISE can be utilized to determine the possible effectiveness of alternative treatments including upper airway surgery and oral devices. DISE cannot be safely performed in patients with American Society of Anesthesiologists class 4, pregnancy, or allergies to sedative agents [17]. A high apnea-hypopnea index (AHI) score is not considered as a contraindication for DISE [17].
Upper Airway Surgery
In a previous systemic review, the plan for OSA surgery was changed in 50.2% of patients after performing DISE, and the changes were mainly related to obstructions in the hypopharynx or larynx compared to awake endoscopy [18]. However, the changes did not automatically lead to favorable surgical outcomes [18]. In a previous study, complete collapse of the lateral pharyngeal wall showed a strong association with the severity of OSA [19]. In addition, improved stability of the lateral pharyngeal wall observed in DISE showed a direct correlation with improved AHI after maxillomandibular advancement procedures [20]. Concentric palatal obstruction or hypopharyngeal-related obstruction was related to worse outcomes after uvulopalatopharyngoplasty [21,22]. Moreover, tongue-related obstruction or complete concentric collapse of the velum was related to worse outcomes in patients who underwent one or more surgical procedures for OSA [23]. Therefore, patients with complete concentric collapse of the pharynx should be treated with PAP rather than with OSA surgery. However, if a patient refuses to undergo CPAP and wants an operation, OSA surgery with tonsillectomy and soft palate surgery, which can sustain the lateral wall of the pharynx, such as suspension lateral pharyngoplasty, relocation pharyngoplasty, expansion sphincter pharyngoplasty, z-pharyngoplasty, and barbed reposition pharyngoplasty can be performed as an alternative treatment. DISE may show obstruction of the laryngeal inlet caused by posterior retraction of the epiglottis, and surgical laser wedge resection can be performed to open the laryngeal inlet [24]. However, as a recent European position paper on DISE indicated, further investigations are required to evaluate in detail whether or not certain DISE findings are related to treatment outcomes [25]. Table 3 summarizes the summarized the recommendation of treatment options from previous studies according to DISE findings. The association between DISE findings and outcomes of OSA surgery may help clinicians select proper candidates for OSA surgery. A previous study evaluated the effectiveness of DISE to determine the surgical plan for single- or multi-level surgeries and upper airway stimulation. They found that the subjects who underwent DISE had a higher success rate for OSA surgery than those who did not (84% vs. 52%, p < 0.001) [26]. In summary, the role of DISE for OSA surgery is to identify localization of obstruction sites, eliminate unnecessary procedures, and improve clinical outcomes.
Mandibular Advanced Device
Mandibular advanced device (MAD) is an effective treatment option for mild to moderate OSA. The jaw-thrust maneuver during DISE is used to predict the effect of MAD. Patients with a substantial improvement in upper airway patency during the jaw-thrust maneuver had better outcomes with MAD [27]. In addition, 97% of the patients who showed increased airway dimensions with MAD during DISE achieved favorable treatment outcomes with MAD [16]. Therefore, MAD may be indicated for patients with improvement in upper airway patency with the jaw-thrust maneuver or MAD during DISE.
Limitation of DISE
There are some limitations of DISE as an evaluation tool for OSA. First, in contrast to polysomnography, DISE does not reflect the information for the entire night. Therefore, the findings in DISE could be a snapshot of the sleep events during the night. Second, unlike SVF, the whole airway cannot be seen at once in DISE. Therefore, DISE could be used complementary to SVF [28]. Lastly, standardization of setting in sedation and evaluation system is limited. Although some hospital-based standardization of sedative agents and the evaluation system was established, considering that DISE reflects the subjective view of the operator through the endoscope, general standardization is necessary.
SUMMARY
Identifying the obstruction sites of the upper airways, with their severity and pattern, in OSA is crucial for determining the treatment plan. DISE is a safe and useful tool to assess dynamic upper airway collapse before surgery. DISE findings are helpful to determine the best treatment option for OSA patients. Although DISE is commonly utilized to aid surgical decision making, it can also assist the optimization of medical therapy, such as MAD.
Acknowledgments
None.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Authors’ Contribution
Conceptualization: Rhee CS. Data curation: Kim JY, Han SC. Investigation: Rhee CS, Kim JW, Kim HJ. Methodology: Kim JY, Lim HJ, Han SC. Project administration: Rhee CS. Resources: Rhee CS, Kim JW, Kim HJ. Supervision: Rhee CS. Validation: Rhee CS, Kim JW, Kim HJ. Visualization: Kim JY, Lim HJ. Writing—original draft: Kim JY, Rhee CS. Writing—review & editing: Rhee CS, Kim JW, Kim HJ.