The Effect of Non-Pharmacological Treatment for Psychophysiological Insomnia on Cardiovascular Autonomic Regulation Assessed Using Heart Rate Variability
Article information
Abstract
Background and Objective
Cardiac autonomic regulation is altered in psychophysiological insomnia. We evaluated whether successful non-pharmacological treatment for psychophysiological insomnia could stabilize cardiac autonomic regulation.
Methods
Subjects were 26 patients with psychophysiological insomnia who underwent four sessions of non-pharmacological treatment. We measured subjects’ heart rate variability (HRV) at baseline and post-treatment. Based on the post-treatment Insomnia Severity Index (ISI) score, we categorized subjects into responder (n = 16, post-ISI < 8) and non-responder (n = 10) groups.
Results
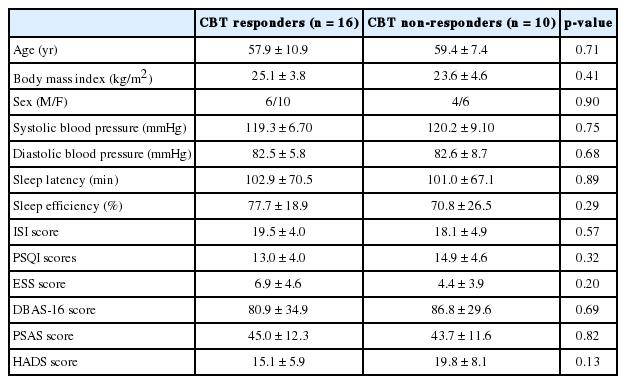
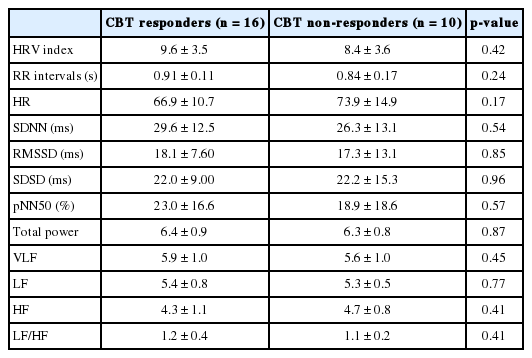
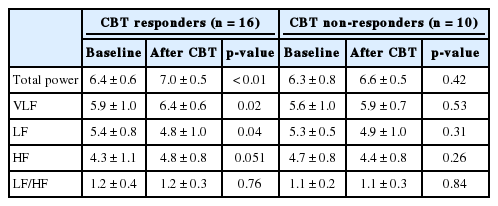
At baseline, we found no significant differences between responder and non-responder groups in age, sex, body mass index, insomnia severity, and features of HRV time and frequency domains. In the responders group, we observed significant increases in the standard deviation of the normal sinus to normal sinus interval (SDNN) (p = 0.02), the proportion of the number of interval differences of successive normal sinus to normal sinus intervals greater than 50 ms by the total number of normal sinus to normal sinus intervals (pNN50) (p = 0.02), total power (p < 0.01), and very low frequency (p = 0.02) and a significant decrease in low frequency (p = 0.04) after successful non-pharmacological treatment for insomnia. However, in the non-responders group, there were no significant changes in HRV features after treatment.
Conclusions
The successful non-pharmacological treatment of insomnia may reduce the risk of cardiovascular complications in patients with psychophysiological insomnia.
INTRODUCTION
Insomnia is defined as difficulty initiating or maintaining sleep, waking up too early, or experiencing non-restorative sleep. Several studies have shown that insomnia is associated with various medical conditions, including hypertension, diabetes, and congestive heart failure.1,2 Furthermore, both insomnia and short sleep duration are associated with increased risk of cardiovascular complications and high mortality rates.3,4 Studies have examined the relationship between insomnia and the risk of cardiovascular complications by measuring heart rate variability (HRV),5–7 which is a non-invasive method for assessing cardiovascular risk.8 This research showed altered cardiac autonomic regulation, as measured using HRV, in patients with psychophysiological insomnia.
Although chronic sleep disturbance may increase the risk of serious health problems, whether improving insomnia will improve health is debatable.9 One recent study found that treating insomnia with hypnotics or non-pharmacological treatment improved patients’ quality of life (QOL).10–13 However, other studies have reported hypnotics decreased the physical aspects of QOL.11 Furthermore, long-term use of hypnotics is associated with various adverse events and, potentially, dependency.
Non-pharmacological therapy is a first-line treatment for primary insomnia,14 and it comprises behavioral, cognitive, and educational components. Most studies have reported that non- pharmacological treatment alone, or in combination with pharmacotherapy, was more effective, with longer lasting effects, than was pharmacotherapy alone.15–17 Moreover, non-pharmacological treatment is safer than medication for treating insomnia. In the present study, we hypothesized that insomnia treatment would reduce the risk of cardiovascular complications, as measured by HRV. This study aimed to test whether successful non-pharmacological treatment of psychophysiological insomnia would normalize cardiac autonomic activity.
METHODS
Subjects
Between May 2009 and November 2010, we recruited subjects from the sleep clinic at the Department of Psychiatry in the Asan Medical Center. All patients had been newly diagnosed with psychophysiological insomnia according to the diagnostic criteria of the International Classification of Sleep Disorders-2nd edition.18 The exclusion criteria were 1) current use of sleeping pills, psychotropic agents, antihypertensive or antihyperlipidemic agents, or medication to treat diabetes; 2) presence of a concurrent major psychiatric condition, such as major depressive disorder or anxiety disorder; and 3) the presence of other, concurrent sleep disorders, such as circadian rhythm sleep disorder, restless legs syndrome (RLS), periodic limb movements during sleep (PLMS) or obstructive sleep apnea syndrome (OSAS). All prospective participants underwent a full history and psychiatric examination by a psychiatrist (sleep specialist). The subjects were asked to complete a sleep diary for a week. To assess patients for sleep disorders such as RLS, PLMS, and OSAS, we utilized sleep questionnaires and/or reports from the patients and their families. We administered the HRV test and various rating scales to the subjects at baseline. These rating scales were the Insomnia Severity Index (ISI),19 the Pittsburg Sleep Quality Scale (PSQI),20 the Epworth Sleepiness Scale (ESS),21 the abbreviated version of the Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS-16),22 the Hospital Anxiety and Depression Scale (HADS),23 and the Pre-Sleep Arousal Scale (PS-AS).24 The subjects underwent four non-pharmacological treatment sessions over an 8-week period, after which we repeated the rating scales and HRV test. This protocol was approved by the Institutional Review Board of the Asan Medical Center.
Non-Pharmacological Treatment
In the present study, cognitive-behavioral treatment (CBT) consisted of four sessions, one every other week over an eight-week period. A psychiatrist (sleep specialist) and a specialized nurse conducted all sessions, individualized for each subject. The first was the baseline session, in which we assessed subjects for sleep and psychiatric disorders and taught them about psychophysiological insomnia and sleep hygiene. Additionally, the subjects were instructed to continue their sleep diaries. During the second session, we analyzed each subject’s sleep diary and conducted their sleep restriction therapy based on information from the analysis. Each subject also received instructions about abdominal breathing. During the third session, we assessed changes in the subject’s sleeping patterns and cognition due to sleep restriction. The subject then received stimulus control therapy and instruction in progressive muscular relaxation. In the last session, we reviewed the changes that occurred during the previous three sessions and gave the subjects paradoxical intention and cognitive therapy and instructions on guided imagery.
Heart Rate Variability Measures
Each subject’s HRV was recorded for 5 min after a rest period of at least 10 min. Electrocardiogram (EKG) signals were continuously monitored and simultaneously sampled using an EKG monitor (LXCJ103, Laxtha, Daejeon, Korea) and the data were analyzed using a data analyzer (CANS 3000, Laxtha). The HRV measurements followed the standards that the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology suggested in 1996.25
We calculated spectral power for four frequency bands: very low frequency (VLF, ≤ 0.04 Hz), low frequency (LF, 0.04–0.15 Hz), high frequency (HF, 0.15–0.4 Hz), and total power (0–0.4 Hz) and performed a natural logarithmic transformation on these HRV parameters. Next, we calculated the LF to HF ratio, expressing it as LF/HF. The time domain variables we calculated were heart rate, HRV index, the standard deviation of the normal sinus to normal sinus interval (SDNN), the square root of the mean of the sum of the squares of differences between adjacent normal-to-normal intervals (RMSSD) the standard deviation of successive differences between adjacent normal-to-normal intervals (SDSD), and the proportion of the number of interval differences of successive normal sinus to normal sinus intervals greater than 50 ms by the total number of normal sinus to normal sinus interals (pNN50).
Statistical Analysis
To compare clinical characteristics between subjects in the CBT responders group and those in the non-responders group, we employed student’s t-test and chi-square analyses. We assessed within-group changes using paired t-tests. Statistical significance was set at p < 0.05 for two-tailed tests. The Statistical Package for the Social Sciences (SPSS), version 12.0 K (SPSS Inc., Chicago, IL, USA), was used to conduct these statistical tests.
RESULTS
We enrolled 46 patients with psychophysiological insomnia who were not taking sleeping pills, psychotropic agents, antihypertensive or antihyperlipidemic drugs, or diabetes medication. Of these 46 subjects, we excluded 20 who did not complete the four-session CBT regimen. We divided the remaining 26 subjects into CBT responders (n = 16) and CBT non-responders (n = 10) based on their post-treatment ISI score (defining “responding” as having a post-treatment ISI < 8).
No significant differences in age, body mass index (BMI), sex, or blood pressure were found between the CBT responder and CBT non-responder groups (Table 1). Likewise, we found no significant between-group differences in baseline reported sleep latency and sleep efficiency, ISI, PSQI, ESS, PSAS, DBAS-16, and HADS scores. Furthermore, the baseline time- and frequency-domain HRV variables did not differ significantly between groups (Table 2). Following treatment, the CBT responder group’s time domain variables (SDNN, p = 0.02; pNN50, p = 0.02) and certain frequency domain variables (total power, p < 0.01; VLF, p = 0.02) significantly increased (Table 3) while LF (p < 0.04) significantly decreased (Table 4) and HF (p = 0.051) marginally increased. However, we observed no significant changes in time or frequency domain variables in the CBT non-responder group (Table 3 and 4).
DISCUSSION
In the present study, SDNN and pNN50 time domain variables increased significantly following successful non-pharmacological treatment. Furthermore, following treatment the frequency domain variables of total power and VLF power significantly increased, while LF power significantly decreased. Our results suggest that successful non-pharmacological treatment for insomnia may reduce the risk of cardiovascular complications.
Research indicates decreased HRV results from increased sympathetic activation and reduced parasympathetic activation.25 Furthermore, studies have shown decreases in time domain variables, such as SDNN, RMSSD, and pNN50, occur in association with an increased risk of cardiovascular complications.8 Of the frequency domain variables, HF correlates highly with parasympathetic tone, and LF reflects sympathetic activity. In contrast, the physiology underlying VLF power is poorly understood.26,27
The human response to chronic sleep disturbance is similar to that for stress and depression.28,29 Sleep deprivation may cause neurotransmitter and neuroendocrine system changes, which the autonomic sympatho-adrenal system and the hypothalamic-pituitary-adrenal axis mediate.30 Moreover, insomnia or sleep restriction correlates with an increased risk of cardiovascular complications.31 Researchers have demonstrated this relationship via the HRV test,5,32–34 an established noninvasive tool for quantifying sympathetic and parasympathetic modulation. Healthy, sleep-deprived subjects show evidence of changes in sympathetic and parasympathetic modulation.35,36 Reportedly, SDNN decreases significantly during sleep in patients with insomnia,5 and a study found lower wake-to-sleep heart rates and lower SDNN in subjects with subjectively-reported insomnia.34 These findings indicate patients with insomnia have detectable, constant sympathetic hyperactivation.33 Furthermore, spectral analysis has revealed significantly increased LF power or significantly decreased HF power in patients with insomnia.5,32 These data indicate chronic sleep disturbance correlates with increased sympathetic nervous system activity and an increased risk for the development of cardiovascular disorders.
In the present study, we observed significant HRV changes in the CBT responders group but no changes in the non-responder group, suggesting successful insomnia treatment reduces sympathetic hyperactivity and stimulates parasympathetic activity. Several studies have investigated hypnotics’ effects on HRV,37,38 though their results were not conclusive. No study has examined the effect of non-pharmacological insomnia treatment on HRV in patients with psychophysiological insomnia. Non-pharmacological treatment in the present study included sleep restriction, stimulus control, cognitive therapy, and relaxation training. We found non-pharmacological insomnia treatment significantly affected HRV of patients in the CBT responder group but did not significantly influence HRV in non-responders. Our findings suggest that successful insomnia treatment may improve patient health. These findings also provide evidence of non-pharmacological treatment’s impact. Electroencephalogram spectral analysis studies have confirmed that CBT is an effective insomnia treatment.39,40 Moreover, reportedly non-pharmacological treatment of post-traumatic stress disorder and other anxiety disorders41,42 has effectively stabilized HRV. Further study is needed on how non-pharmacological treatment affects hyperarousal states in insomnia and other psychiatric disorders.
The present study is limited by its small sample size. It may have been underpowered for detecting significant group differences. The small sample size resulted from our exclusion of patients taking medications, including sleeping pills, psychotropic agents, and antihypertensive or antihyperlipidemic agents, to rule out any medication effects on cardiac autonomic activity.38,43 Another study limitation is the lack of normal control subjects. We did not perform nocturnal polysomnography (NPSG) in this study. A primary objective of NPSG is the exclusion of subjects with sleep disorders, such as RLS, PLMS, and OSAS. OSAS in particular can influence cardiac autonomic activity.44 However, patients with psychophysiological insomnia do not routinely undergo NPSG, and we found no significant between-group differences in OSAS risk factors, such as sex, age, and BMI. In conclusion, the successful non-pharmacological treatment of insomnia may reduce the risk of cardiovascular complications in patients with psychophysiological insomnia.
Acknowledgments
This study was supported by a education & research fund (2010-488) from the Asan Institute for Life Sciences, Seoul, Korea.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.