INTRODUCTION
Sleep is essential for maintaining normal body function. The appropriate amount of sleep is important, but the continuity of sleep is also important [1]. Sleep fragmentation (SF) can be defined as disrupted sleep with abrupt changes in electroencephalogram (EEG) during sleep, which is known as sleep arousal. SF can be caused by internal factors (e.g., sleep apnea, restless leg syndrome, and pain) or external factors such as noise and temperature [2,3]. Clinically, SF has been reported to occur at high frequency in patients with osteoarthritis and arthritis, and frequent SF is an important factor in reducing sleep quality in these patients. In particular, SF is common in patients with obstructive sleep apnea (OSA) [4] and frequently occurs in patients with lung diseases such as chronic obstructive pulmonary disease, nighttime asthma, narcolepsy, and Parkinson’s disease. SF causes a variety of clinical problems; in animal and human studies, acute and chronic SF are associated with increased blood pressure, vascular endothelial dysfunction and changes in blood vessel structure, increased resistance to leptin and insulin, weight gain, decreased blood-brain barrier function, and cancer cell growth [5,6].
Inflammasomes are multi-protein complexes that play a key role in the innate immune system. They comprise an intracellular sensor, a NOD-like receptor (NLR), procaspase-1, and ASC, an adapter protein [7]. Inflammasome activation promotes autocatalytic activation of a cysteine protease, caspase-1, which activates the cytokines pro-IL-1β and IL-18. NLR protein-3 (NLRP-3) is a representative inflammasome that consists of NLRP-3, ASC, and pro-caspase-1; this complex is essential for producing mature IL-1β in response to a variety of signals. NLRP-3 is activated by danger-associated molecular patterns, including endogenous risk signals such as bacterial toxins, pathogen-associated molecular patterns, and adenosine triphosphate (ATP). ATP outside the cell binds to the P2X7 receptor and promotes K+ release. This K+ release reduces intracellular K+ levels, which induces oligomerization of NLRP-3 and activates it [8]. NLRP-3 can also be activated by other endogenous stress-related risk signals such as monosodium urate crystals, β-amyloid, and reactive oxygen species (ROS) [9-11]. NLRP-3-dependent IL-1β has been reported to contribute to the development of insulin resistance [12] and is associated with impaired cognitive function [13,14].
SF resulted in cognitive and memory dysfunction by increasing nicotinamide adenine dinucleotide phosphate oxidase activity [15] and down-regulating the proteins that account for synaptic neurotransmission [16]. However, the exact mechanism by which SF impairs cognitive and memory function remains unresolved. Because IL-1β is associated with cognitive impairment and because IL-1β expression and its activator can be up-regulated in stress conditions, we hypothesized that SF could increase IL-1β levels in the hippocampus by activating NLRP-3 inflammasome. That is, increased IL-1β might have an important role in memory and cognitive impairment. To test this hypothesis, we investigated whether SF activated NLRP-3 inflammasome in the hippocampus and the possible mechanisms of this activation.
METHODS
Animals
We used 7-week-old male Wistar rats (Orient Bio, Seongnam, Korea) in this study. All animals were maintained in a temperature-controlled facility with alternating 12-h cycles of light and dark (light on at 8:00 am); the animals had free access to food and water. We randomly assigned 48 rats to one of the following groups: 4 days of SF (n = 12), 8 days of SF (n = 12), 4 days of exercise control (EC; n = 12), and 8 days of EC group (n = 12). We also used an additional 10 rats for EEG measurements during SF (n = 5) and EC (n = 5).
Sleep Recording and Analysis Conditions
We recorded sleep EEGs to evaluate the adequacy of the sleep segmentation model. We implanted two pairs of EEG and one pair of electromyography (EMG) electrodes in the rats, using isoflurane (Baxter, San Juan, Puerto Rico, USA) as an inhalation anesthetic. The induction concentration was 5%, and the maintenance concentrations were 2–2.5%. We inserted a pair of EEG electrodes (Pinnacle Technology Inc., Lawrence, KS, USA) into the frontoparietal and lateral lobes and a pair of EMG electrodes into the neck muscles, fixing them with a non-absorbable seal. After we connected the head mount to the electrodes, we firmly fixed them to the rat’s head with dental acrylic. We sutured the incisions and administered antibiotics intraperitoneally for three days after surgery to prevent inflammation. After the operation, we dressed the wound area for several days and obtained a sleep EEG after 14 days. We amplified EEG and EMG signals with a preamplifier (Pinnacle Technology) and Sirenia software (Pinnacle Technology) to analyze the sleep stage. We divided sleep into three stages, awake, non-rapid eye movement (NREM), and rapid eye movement (REM), according to the amplitude and spectral analysis of the EEG and EMG waveforms; we set the duration of one epoch at 10 seconds. We recorded and analyzed 24 hours of EEG at baseline and after 4 or 8 days of SF or EC. We defined the frequency range for each waveform as delta 0.5–4 Hz, theta 4–8 Hz, and alpha 8–13 Hz. We analyzed the percentage of sleep time by stage, number of bouts, and length of bouts during the entire recording time, defining about as at least two consecutive 10-s epochs for a given state that ended with any single state-change epoch.
SF and EC Conditions
We used a rat forced-locomotion wheel system (80805A; Lafayette Instrument Company, Lafayette, IN, USA) for SF and EC. For SF, the wheel-based device was operated with a 90 sec off/30 sec on cycle for 6 h/day to mimic the arousal frequency of severe OSA. The speed was 4 m/min considering that the average speed of a rat is 7 cm/s. The conditions for EC were 10 min on/30 min off for 6 h/day, which allowed for longer periods of undisturbed sleep. SF and EC were performed from 10:00 am to 4:00 pm during the entire experimental periods.
Western Blotting
After 4 or 8 days of SF or EC, the mice were anesthetized with 5% isoflurane, and their hippocampi were collected on ice. The tissue was immediately immersed in liquid nitrogen and stored at -80°C. The hippocampal tissues were homogenized in RIPA buffer (Sigma, St. Louis, MO, USA) supplemented with protease inhibitors. We quantified total protein concentrations with a BCA assay kit (Pierce, Rockford, IL, USA). For Western blotting, we loaded 50 μg onto a 10% SDS-PAGE gel and then transferred the protein to a PVDF membrane at 300 mA for 1 hour. After electrophoresis, we confirmed the protein bands by Ponceau S staining. We washed the membranes with Tris-buffered saline with Tween 20 and blocked them with 5% skim milk for 1 hour to block nonspecific reactions. We diluted primary antibodies as follows: rabbit polyclonal anti-caspase-1 (1:1000; Abcam, Cambridge, MA, USA), cleaved caspase-1 (1:500; Novus Biologicals, Littleton, CO, USA), rabbit polyclonal anti-ASC (1:1000; Abcam), rabbit polyclonal anti-NLRP-3 (Abcam), rabbit polyclonal anti-thioredoxin-interacting protein (TXNIP) (1:1000; Santa Cruz Technologies, Dallas, TX, USA), and rabbit polyclonal anti-IL-1β (1:500; Abcam). The secondary antibody was goat anti-rabbit HRP-conjugated antibody in 5% skim milk at 1:5000 for 1 hour at room temperature. We used β-actin (1:20,000; Bioworld Technology, St. Louis Park, MN, USA) as an internal control. We detected the signals with an ECL solution. We then developed the protein bands on X-ray film and converted them into image files with a scanner, and we quantified the band density with ImageJ. We normalized the intensity of each protein band to β-actin.
RESULTS
Wheel-Based System Effectively Fragmented Sleep in Rats
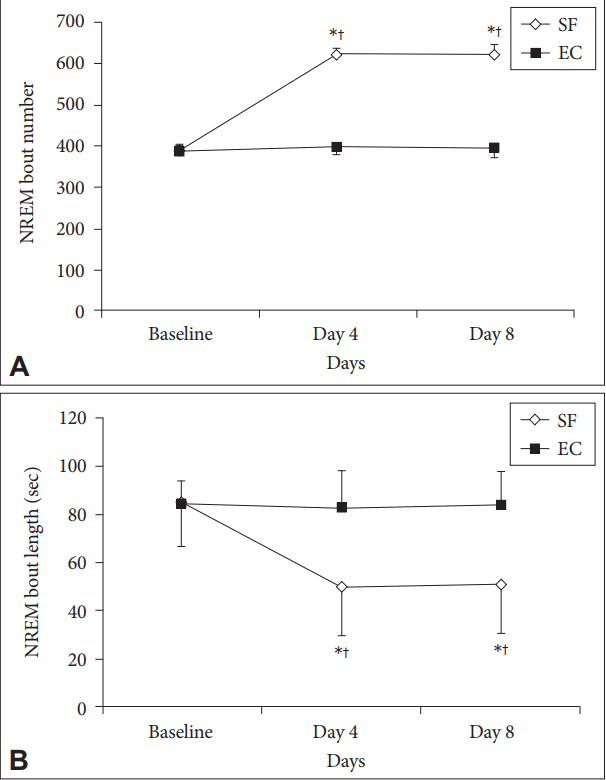
Both the SF and EC groups showed significantly higher percentages of time awake at day 4 than at baseline (Fig. 1A), although the amount of time spent awake did not differ significantly between the groups. Throughout the experiment, the percentages of time spent in NREM and REM sleep did not differ significantly between the SF and EC groups or between baseline and follow-up (Fig. 1B and C). The SF group had more NREM but shorter bouts, which indicated highly fragmented NREM sleep (Fig. 2). The EC group had no significant difference in number of NREM bouts or NREM bout length compared with baseline, implying preserved sleep continuity in the EC group.
SF Induced Up-Regulation of the NLRP-3 Complex
We examined the expression levels of NLRP-3 and associated proteins by Western blot (Fig. 3A and C), and NLRP-3 expression was highest in the 4-day SF group. In the 8-day SF group, NLRP-3 expression was slightly lower than in the 4-day SF group but higher than in the 4-day and 8-day EC groups. Expression patterns of caspase-1 and ASC, which form the NLRP-3 complex, were similar to that of NLRP-3. Levels of mature IL-1β, which is generated by NLRP-3 activation, were significantly higher in the 4-day and 8-day SF groups than in the corresponding EC groups, and expression of cleaved caspase-1, which indicated caspase-1 activation, showed the same pattern. Because it is known that sleep disturbance may increase ROS, we examined the expression of TXNIP, which binds NLRP-3 and activates it under oxidative stress conditions [17]. TXNIP expression was also higher in the SF groups than the EC groups (Fig. 3B and C).
SF Increased Lipid Peroxidation
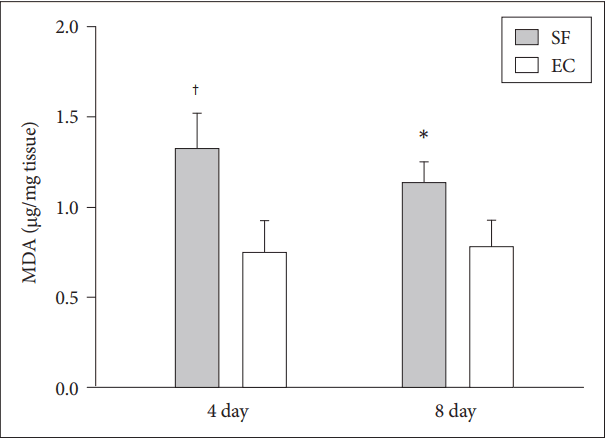
Next, we investigated the levels of MDA as a lipid peroxidation marker to confirm whether oxidative stress was actually higher in hippocampi subjected to SF, and at 4 days, MDA was higher in the SF group than in the EC group. MDA levels were slightly lower in the 8-day SF group than in the 4-day SF group but still higher than in the 8-day EC group (Fig. 4).
DISCUSSION
In this study, we investigated the effects of 4 days and 8 days of SF on NLRP-3 activation in rat hippocampi using a forced walking wheel. Four days and 8 days of SF significantly increased NLRP-3 inflammasome expression compared with EC. We also found that higher NLRP-3 inflammasome expression in the SF groups was associated with increased oxidative stress.
Mounting evidence has revealed a significant relationship between sleep disturbance and IL-1β. Zielinski et al. [18] reported that experimentally induced sleep restriction elevated the mRNA expression of IL-1β in several regions of the rat brain. Moreover, according to a recent study that examined the modulating effect of NLRP-3 on spontaneous sleep and homeostatic sleep response following sleep deprivation (SD), NLRP-3 knockout (KO) mice showed no significant differences in IL-1β expression in the somatosensory cortex as well as caspase-1 activity following 6 h of SD compared with baseline, whereas wild-type mice showed elevated caspase-1 activity and NLRP-3, ASC, and IL-1β expression [19]. In the same study, NLRP-3 KO mice also showed reduced NREM sleep during the light period, and their homeostatic sleep responses following SD were attenuated. These findings suggest NLRP-3 activation as an important molecular mechanism for regulating SD-induced sleep response. Our study is important in that acute SD is known to induce NRLP-3 inflammasome activation in brain regions including the hippocampus, but it is not known in chronic SF conditions, which are more common in real life.
IL-1β has been associated with decreased cognitive function under various conditions; the association between IL-1β levels and hippocampus-dependent memory has a U-shape. Low IL-1β promotes long-term potentiation and memory formation, whereas high IL-1β reduces memory consolidation [13,20-22]. IL-1β levels have been reported to increase in the hippocampal tissues of obese rats fed high-fat diets, which is associated with memory dysfunction [14]. However, the same study showed that IL-1 receptor antagonists in the central nervous system restored memory loss, which indicated that IL-1β caused hippocampusdependent memory loss. Several molecular and physiological mechanisms have been suggested to induce hippocampus-dependent memory deficits through IL-1β. IL-1β inhibits glutamate release and reduces calcium influx in the hippocampus synaptosome [23]. It may also affect memory by modulating brain-derived neurotrophic factor (BDNF), which plays an important role in synaptic plasticity and long-term memory. According to a previous study, administering systemic IL-1β or lipopolysaccharide, which can increase IL-1β, resulted in down-regulation of BDNF mRNA in rat hippocampal regions subjected to 6 h of social isolation [24]. In contrast, an IL-1 receptor antagonist prevented both down-regulation of BDNF expression and memory impairment in rats subjected to social isolation stress [25].
In addition to a role in memory and cognitive function, NLRP-3 inflammasome plays an important role in various metabolic diseases. Glucose tolerance and insulin sensitivity have been reported to improve after a high-fat diet was fed to mice that had the NLRP-3 cytoplasmic receptor or ASC and caspase-1 genes knocked out [12]. NLRP-3 inflammasome may also play an important role in atherosclerosis. Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of lipids and immune cells in blood vessels [26]. NLRP-3 activation promoted hyperhomocyteinemia-aggravated inflammation in macrophages and concurrent atherosclerosis in apolipoprotein E (apoE)-deficient mice [27]. Moreover, inhibiting IL-1β in apoE-deficient mice that had atherosclerosis due to hypercholesterolemia was reported to reduce the incidence of atherosclerotic lesions [28].
Because previous studies have reported significant associations between sleep disturbance and oxidative stress and because ROS is known to affect NLRP-3 activation, we examined whether NLRP-3 activation in the SF rat groups was associated with oxidative stress. Lipid peroxidation in the hippocampus, as demonstrated by MDA levels, was significantly higher in the SF groups than in the EC groups, indicating increased oxidative stress in the SF groups. In addition, we observed significantly more up-regulation of TXNIP in the SF groups, which binds to NLRP-3 and activates it under oxidative stress [17]. These findings suggest that SF-induced elevation of oxidative stress activated NLRP-3 inflammasome in rat hippocampi. This possibility could be confirmed pharmacologically by using ROS scavengers.
In this study, we confirmed that SF increased NLRP-3 inflammasome expression and activation in rat hippocampal tissues. Although previous studies have shown that IL-1β plays an important role in hippocampus-dependent memory and cognitive function, this study did not include behavioral tests to assess such cognitive functions. Therefore, future behavioral and mechanistic studies are required to elucidate the role of SFactivated NLRP-3 on hippocampus-dependent cognitive function.