AbstractBackground and ObjectiveSimulated altitude as a model for hypoxia has shown inconsistent results in terms of impaired cognition. We hypothesized that preserved periods of stable sleep even under hypoxia could explain stable cognitive function. Delta spectral power on electroencephalography during stable sleep as well as high frequency coupling on the electrocardiogram-based spectrogram was adopted as measures of sleep quality.
MethodsEleven healthy, non-smoking subjects (7 men, 27 ± 1.5 years) were exposed to 9 hours of continuous hypoxia for 13 consecutive nights. Polysomnography was done at baseline and during 3 time points, at night 3, 7, and 14. In each study, delta spectral power was obtained during stable N2 and N3 sleep. Stable sleep was defined when there was no significant fragmentation in electroencephalography and fluctuation in electromyography and cardiorespiratory signals. The time threshold was 2 or 5 continuous minutes for N2 and 2 minutes for N3. The amount of high frequency coupling for the sleep period on the electrocardiogram-based spectrogram was computed. Randomized block ANOVA was used with electroencephalography delta power and high frequency coupling as dependent variables with post hoc Tukey test.
ResultsDelta spectral power during stable sleep was not significantly different across the entire hypoxic exposures (p = 0.98 for N2; p = 0.32 for N3). High frequency coupling was different between pre-exposure and mid-exposure (night 7; 52.5 ± 23.6% vs. 39.0 ± 16.7%, p = 0.02) but returned to the baseline level at the post-exposure (night 14; 45.4 ± 18.2%, p = 0.39).
INTRODUCTIONSleep contributes to memory and cognitive function through modulation of neural plasticity and synaptic homeostasis.1,2 Numerous clinical and experimental data have demonstrated that disrupted or diminished sleep leads to cognitive impairment. Therefore, good quality and adequate quantity of sleep is essential to normal waking cognitive performance. Beside acute or chronic sleep restriction in modern life, sleep-disordered breathing is a highly prevalent and well-described example of qualitative impairment of sleep leading to cognitive dysfunction.3,4 Mechanisms underlying its association with cognitive impairment could be inferred from the immediate results of sleep-disordered breathing. Two prominent effects are sleep fragmentation and nocturnal hypoxia.5,6 These are known to activate cascades of events such as hemodynamic (blood pressure) fluctuations, oxidative injury, sympathetic over-activity, systemic inflammation, and glucose dysmetabolism.7 All of these consequences have been implicated as possible contributors to impaired cognitive function.8
The relative contributions of sleep fragmentation and nocturnal hypoxia are elusive, because sleep fragmentation and hypoxia invariably occur simultaneously in both human cases and animal models of sleep disordered breathings.9 To delineate the individual effects, we previously applied a simulated altitude nocturnal hypoxia model to young healthy adults and demonstrated that the short term (2–4 weeks) hypoxia did not cause significant deficit in subjective or objective vigilance and working memory.10,11 Based on this finding, we hypothesized that preserved sleep quality contributes to the maintained cognitive function in this model.
The conventional method for evaluating sleep quality is an assessment of sleep architecture determined by visual scoring of electroencephalography (EEG) that measures the amount of sleep stages or an arousal index.12
In previous studies, an increase of light sleep and arousals was observed during exposure to nocturnal hypoxia,10,11 which does not support our hypothesis. However, a limitation of EEG-based sleep quality assessment is that sleep staging is constrained by the amplitude and morphology of EEG waves and is not consistently correlated with perceived sleep quality.12,13 Therefore we adopted two alternative metrics of sleep quality to test our hypothesis. Power spectral analysis of the EEG has been previously used to examine human sleep quality and may provide a more sensitive measure of sleep stability in subjects exposed to nocturnal hypoxia.14–16 Another approach is an analysis of cardiopulmonary coupling (CPC), the electrocardiogram (ECG)-based spectrogram.17 As the CPC technique is based on a continuous ECG signal, it is an EEG-independent, convenient and cost-effective approach with sufficient signal-to-noise ratio. Besides, it is automated and scorer-independent. CPC analysis employs Fourier-based methods to analyze the degree of coupling between two physiologic streams, autonomic drive from heart rate variability, and respiratory drive from ECG-derived respiration.17
CPC outputs the relative amount of stable and unstable NREM sleep. High frequency coupling (HFC) is the biomarker of stable sleep, characterized by strong sinus arrhythmia/vagal dominance, paucity of phasic EEG complexes and arousals, and stable breathing periods in those with sleep apnea. Low frequency coupling (LFC) is unstable sleep, and is associated with cyclic variation in heart rate, fluctuating tidal volumes, and abundant phasic EEG complexes and arousals. We have previously shown that HFC and LFC periods correlate with periods of cyclic alternating pattern (CAP) and non-CAP respectively, but not with conventional EEG sleep stages.17,18 Increased LFC and reduced HFC is also seen in fibromyalgia and depression.19,20 Our hypothesis was that delta power and HFC as a proportion of the sleep period would be preserved at altitude, providing one explanation for maintained cognitive performance in our hypoxia models.
METHODSSubjects, Hypoxic Exposures, Polysomnography, and Cognitive AssessmentThis is a secondary analysis of previously published studies. The details for subject characteristics, nocturnal hypoxia model, polysomnography and assessment of sleepiness, attention and working memory were described elsewhere.10 In brief: eleven healthy subjects (7 men, 27.0 ± 1.5 years old, body mass index of 23 ± 0.9 kg/m2) without significant medial and sleep disorders were recruited. Subjects were exposed during sleep to nine hours of continuous mildly hypocapnic hypoxia. Initial acclimatization consisted of the first night at sea level, the second at 7,700 feet and the third at 10,000 feet and then the experimental hypoxic condition was given for 14 consecutive nights at 13,000 feet using a normobaric “altitude tent system” (Colorado Altitude Training, Colorado Springs, Colorado, USA). Subjects were at sea level during the day. FiO2 (0.13) and CO2 (0.1–0.52%) level was independently verified. Standard polysomnography using Embla (Embla, Denver, Colorado, USA) system was performed at baseline and 3 points during the course of the study (night 3, night 7, and night 14). Polysomnography included recording the EEG, electrooculogram, chin and tibialis electromyogram, thermistor, nasal pressure, thoracic and abdominal effort, ECG, and finger oxymetry. Conventional sleep staging and events scoring including arousal and sleep disordered breathings followed the standard methods.10 Vigilance and working memory assessment consisted with a 10-minute verbal 2-back task and the Psychomotor Vigilance Task (PVT). Performance was measured during the daytime between 08:00 and 10:00 hours to minimize circadian effect at four time points (day 4, 8, 9 and 15). The number of lapses on the PVT and percentage of correct response and mean response time on 2-back task were the primary variables of functional status. Besides, subjective sleepiness and mood was assessed on a scale of 0 (very sleepy or depressed mood) to 10 (usual sleepy or normal mood state).
Delta Spectral Power and Cardiopulmonary Coupling AnalysisDelta spectral power was performed using commercially available polysomnography software, RemLogic 1.0 (Embla, Denver, Colorado, USA) at four time points at the baseline and night 3, 7, and 14. Each time point corresponded to preexposure, early-acclimatized, midexposure and postexposure. Spectral power analysis was performed in the central electrode (C4-A1) during stable N2 and N3 sleep using ‘sleep power band analysis’ (RemLogic 1.0) that shows the relative contributions of each EEG spectrum in the given period. Spectral power band consisted with delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–14 Hz), beta (14–25 Hz), and higher bands (> 25 Hz). The relative power in the delta band was the primary measure of interest for analysis and expressed as percentage (%). Stable sleep was defined as sleep periods with significant fragmentation of the EEG (no wake epochs or respiratory arousals) and fluctuations in electromyography and cardiorespiratory signals (Fig. 1). The time threshold was two or five continuous minutes for N2 and 2 minutes for N3.
Details of our CPC method have been published.17 In brief, using a single lead ECG, an automated beat detection algorithm detects beats and classifies them as either normal or ectopic. The algorithm determines amplitude variations in the QRS complex that occur due to shifts with respiration in the cardiac electrical axis relative to the electrodes, and due to changes in thoracic impedance as the lungs fill and empty. Fluctuations in the mean cardiac electrical axis (typically between 1 degree and 12 degrees peak-to-peak) correlate with phasic changes in the respiratory cycle. From these amplitude variations a surrogate ECG derived respiratory signal (EDR) is obtained. A time series of normal-to-normal sinus (NN) intervals and the time series of the EDR associated with these NN intervals is then extracted from the RR interval time series. Outliers due to false or missed R-wave detections are removed using a sliding window average filter with a window of 41 data points and rejection of central points lying outside 20% of the window average. The resulting NN interval series and its associated EDR are then cubic spline resampled at 2 Hz. The cross-spectral power and coherence of these two signals are calculated over a 1024 sample (8.5 minute) window using the Fast Fourier Transform applied to the 3 overlapping 512 sample subwindows within the 1024 coherence window. The 1024 coherence window is then advanced by 256 samples (2.1 minutes) and the calculation repeated until the entire NN interval/EDR series is analyzed. For each 1024 window the product of the coherence and cross-spectral power is used to calculate the ratio of coherent cross power in the low frequency (0.01–0.1 Hz) band to that in the high frequency (0.1–0.4 Hz) band. A preponderance of power in the low frequency band tends to be associated with periodic sleep behaviors regardless of etiology, while excess power in the high frequency band is associated with physiologic respiratory sinus arrhythmia and stable/deep sleep. CPC spectrograms reveal that the LFC and HFC regimes closely follow CAP scoring.
Specifically, LFC is generally associated with CAP and HFC with non-CAP. It is also observed that the ratio of power in the very low frequency (0–0.01 Hz) to the combined power in the low and high frequency bands may be used to estimate periods of wake or REM sleep, where a preponderance of power in the very low frequency band is associated with wake/REM periods. Using appropriate thresholds for the power ratios in these bands, sleep demonstrating stability and HFC, instability and LFC coupling, and wake/REM states can be identified (Fig. 2). A further characterization of the low-frequency coupled state is performed by determining the % (time) demonstrating elevated-LFC power. This category of coupling is strongly correlated (> 0.8) with apneas and hypopneas scored in the PhysioNet Sleep Apnea Database (http://www.physionet.org/physiobank/database/apnea-ecg/).21 It provides an objective method to introduce a severity dimension to LFC. It does not necessarily signify apneas per se, as arousals due to other reasons may appear indistinguishable. HFC was a variable of primary interest.
Statistical AnalysisMeans and standard deviations were summarized for delta spectral power and CPC variables. Individual responses to nocturnal hypoxia and cognitive performance is known to be significantly different between subjects. Therefore, randomized block analysis of variance was used to assess significance difference between protocol time points with EEG delta power and HFC as dependent variables. Subject and time points of sleep study are parameters for ‘block’. Post hoc Tukey test was implemented for multiple comparisons. Statistical analysis was performed by using SPSS software (SPSS 12.0, SPSS Inc., Chicago, IL, USA). Null hypothesis was rejected at p-value < 0.05.
RESULTSSleep Quality during Nocturnal HypoxiaSleep indices from the CPC analysis are presented in Table 1. The amount of HFC, i.e., stable sleep, changed across the entire hypoxic exposures (p = 0.02). HFC was different between pre-exposure and mid-exposure (night 7; 52.3 ± 23.6% vs. 39.0 ± 16.7%, post hoc Tukey test, p = 0.02) but returned to the baseline level at the post-exposure (night 14; 45.4 ± 18.2%, p = 0.39). Contrary to the change in HFC, slow wave sleep decreased continuously along the hypoxic exposure, although it was not statistically significant (7.6 ± 5.7% vs. 4.7 ± 2.6%, p = 0.19). Among conventional sleep architecture parameters, the other significant differences across the protocol were in total sleep time (baseline to early acclimatization only), stage 1 sleep (baseline to early acclimatization only), and the American Academy of Sleep Medicine arousal index (significantly different from baseline to early acclimatization). Breathing during sleep is highly susceptible to hypoxic exposures. Respiratory disturbance index increased immediately after hypoxic exposures. It was significantly higher across the hypoxic period than the baseline (p = 0.002)(Table 1). There was no significant difference in sleep efficiency or REM sleep measures across the study duration (Table 1). Delta spectral power band analysis showed maintenance of relative delta power during hypoxic exposures. Relative delta spectral power during stable sleep was not significantly different across the entire hypoxic exposure (p = 0.98, 0.25 for N2; p = 0.66 for N3).
Subjective Symptoms and Cognitive PerformanceAs reported before, there were no significant differences from the baseline across the period of exposure in any of the following variables: subjective assessment of state (a composite of mood and sleepiness), 2-back mean reaction times, percentage of correct responses, and the lapses on PVT lapses (Table 1). Other variables from the PVT including mean, median, 10% slowest reaction times or false starts did not change along the hypoxic exposure.
DISCUSSIONEEG spectral analysis and ECG-derived sleep spectrogram based on CPC as a new metric of sleep quality demonstrated different findings from conventional sleep scoring. The key findings of this report are: 1) Delta spectral power band did not change across the hypoxic exposures despite hypoxia-related sleep-disordered breathing. This trend runs parallel to observed cognitive performance. 2) The amount of HFC as an index of sleep quality decreased during the first half of hypoxic exposure but recovered. On the contrary, the amount of slow wave sleep tended to continuously decrease along hypoxic exposures.
These results suggest that the underlying mechanism of preservation of cognitive function across hypoxic exposures is through preserved sleep quality despite the expected induction of altitude-induced sleep apnea.10 This could be explained by preserved delta power. Delta activity on the EEG is regarded as the most accurate biomarker of the homeostatic regulation of sleep, and parallels changes in synaptic plasticity and strength during sleep.22,23 Even under hypoxic conditions, homeostatic regulation of sleep brain function remained relatively intact, at least during stable NREM sleep.
Preserved sleep stability might also explain the maintenance of cognitive performance under hypoxic condition. Though HFC initially decreased during the hypoxic exposure, it returned to the baseline level by the end of the hypoxic period. The initial reduction was probably due to sleep apnea expanding the LFC zone, with a return to baseline as acclimatization occurred. Our prior results suggest that low and high frequency coupled states occur in health and disease, with the high frequency dominating in health.17,19,20,24 Sleep is composed of cyclic alternation between stable and unstable sleep periods, as amply demonstrated by CAP/non-CAP periods. What we are measuring with CPC technique is not simply heart rate variability, but coupled interactions between respiratory and autonomic systems that are strongly modulated by sleep. The CPC technique allows tracking adaptations that occur during the course of a disruptive stimulus or condition. This adaptability may also contribute to the preserved cognitive function seen in this study. On the other hand, slow wave sleep, a conventional metrics of sleep stability, tended to decrease across the exposure (Table 1). These different approaches to characterizing sleep quality probably have complementary value in different clinical conditions.
In summary, we demonstrate that delta power and CPC based sleep quality estimates are preserved during altitude exposure. The result may explain preserved cognition in our study population. Application to other populations may help explain why some individuals with sleep apnea/nocturnal hypoxia have cognitive dysfunction, while others do not.
AcknowledgmentsGrant support: Grants from the National Institutes of Health Heart Lung and Blood Institute: K23HL004457 and RC1HL099749 (RJT), the G. Harold and Leila Y. Mathers Foundation, the NIH-sponsored Research Resource for Complex Physiologic Signals (UO1EB008577) (need to add GCRC).
REFERENCES3. Nowak M, Kornhuber J, Meyrer R. Daytime impairment and neurodegeneration in OSAS. Sleep 2006;29:1521-30.
4. Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol 2005;98:2226-34.
5. Thomas RJ. Sleep fragmentation and arousals from sleep-time scales, associations, and implications. Clin Neurophysiol 2006;117:707-11.
7. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47-112.
8. Veasey S. Insight from animal models into the cognitive consequences of adult sleep-disordered breathing. ILAR J 2009;50:307-11.
9. Rees K, Spence DP, Earis JE, Calverley PM. Arousal responses from apneic events during non-rapid-eye-movement sleep. Am J Respir Crit Care Med 1995;152:1016-21.
10. Thomas RJ, Tamisier R, Boucher J, Kotlar Y, Vigneault K, Weiss JW, et al. Nocturnal hypoxia exposure with simulated altitude for 14 days does not significantly alter working memory or vigilance in humans. Sleep 2007;30:1195-203.
11. Weiss MD, Tamisier R, Boucher J, Lynch M, Gilmartin G, Weiss JW, et al. A pilot study of sleep, cognition, and respiration under 4 weeks of intermittent nocturnal hypoxia in adult humans. Sleep Med 2009;10:739-45.
12. Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, Illinois: American Academy of Sleep Medicine; 2007.
13. Armitage R, Trivedi M, Hoffmann R, Rush AJ. Relationship between objective and subjective sleep measures in depressed patients and healthy controls. Depress Anxiety 1997;5:97-102.
14. Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol 1981;51:483-95.
15. Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep 2004;27:774-83.
17. Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep 2005;28:1151-61.
18. Terzano MG, Parrino L. Origin and Significance of the Cyclic Alternating Pattern (CAP). REVIEW ARTICLE. Sleep Med Rev 2000;4:101-23.
19. Thomas RJ, Mietus JE, Peng CK, Goldberger AL, Crofford LJ, Chervin RD. Impaired sleep quality in fibromyalgia: Detection and quantification with ECG-based cardiopulmonary coupling spectrograms. Sleep Med 2010;11:497-8.
20. Yang AC, Yang CH, Hong CJ, Tsai SJ, Kuo CH, Peng CK, et al. Sleep state instabilities in major depressive disorder: detection and quantification with electrocardiogram-based cardiopulmonary coupling analysis. Psychophysiology 2010.
21. Thomas RJ, Mietus JE, Peng CK, Gilmartin G, Daly RW, Goldberger AL, et al. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep 2007;30:1756-69.
Fig. 1.Stable NREM sleep. According to the pre-defined criteria of stable sleep, EEG shows no fragmentation with absence of wake or arousals, stable respiration and no phasic EMG activity during this 5-minute period. Sleep power band (upper right panel) is acquired from right central EEG (C4-A1). Sleep stages across the night is shown in upper left panel. EEG: electroencephalography, EMG: electromyography 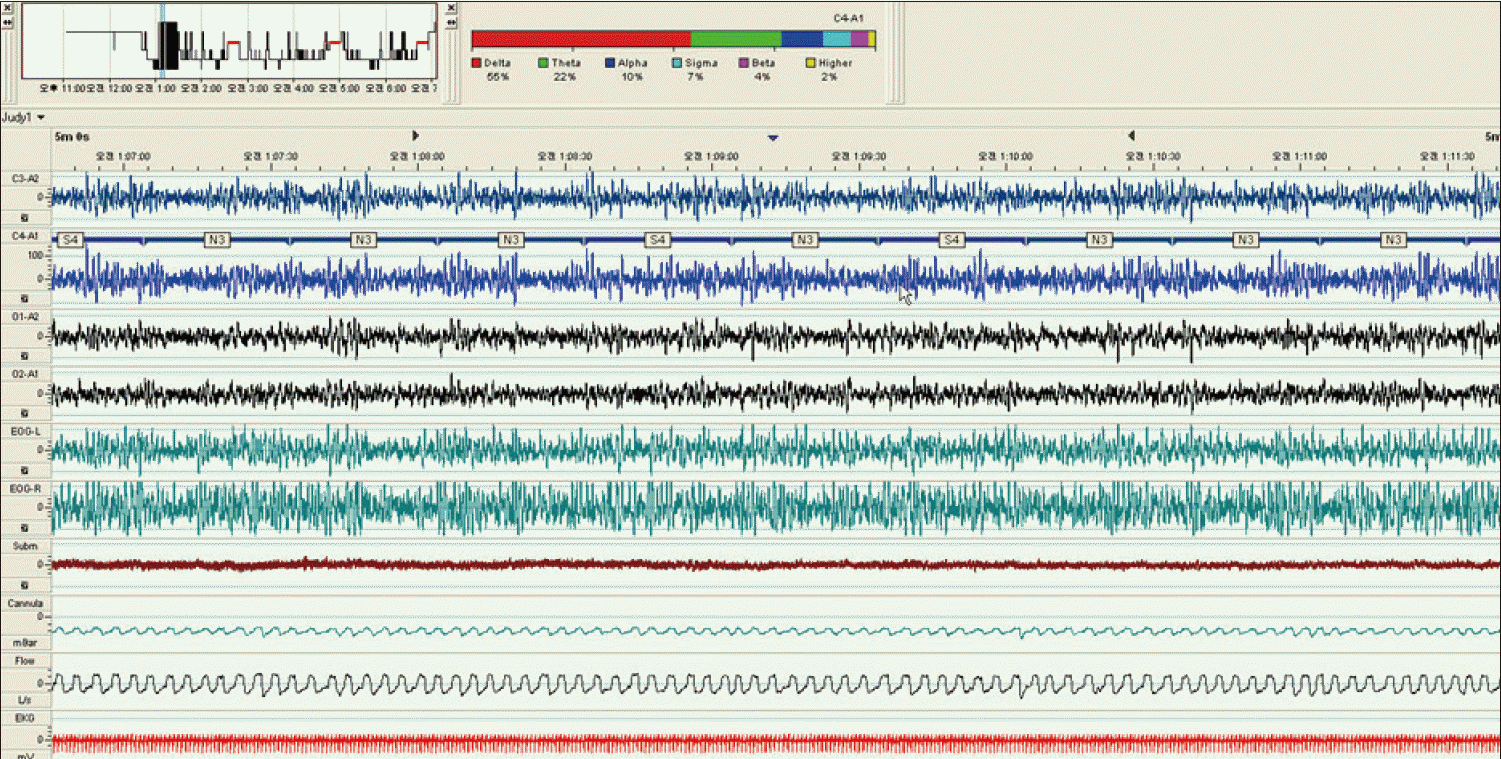
Fig. 2.The normal sleep spectrogram. Cardiopulmonary coupling analysis in a 22-year-old healthy woman. The upper two panels show from top to bottom: conventional sleep stage scored in 30-second epochs and the ratio of low-frequency (0.01–0.1 Hz) to high-frequency (0.1–0.4 Hz) coherent cross power (Lo/Hi Ratio) used to detect sleep state. The bottom panel shows the cardiopulmonary coupling spectrogram across 7 hours of sleep in which the magnitude of the coherent cross power at each frequency is indicated by the height of the peak. The sleep spectrogram reveals spontaneous switching between high-frequency and low-frequency coupled states represented by the 2 distinct bands of spectrographic peaks. High frequency coupling (arrow) denotes stable sleep. W: refers to wake, R: rapid eye movement sleep, C: EEG-cyclic alternating pattern, NC: EEG-noncyclic alernating pattern. Lo: low, Hi: high, EEG: electroencephalography. 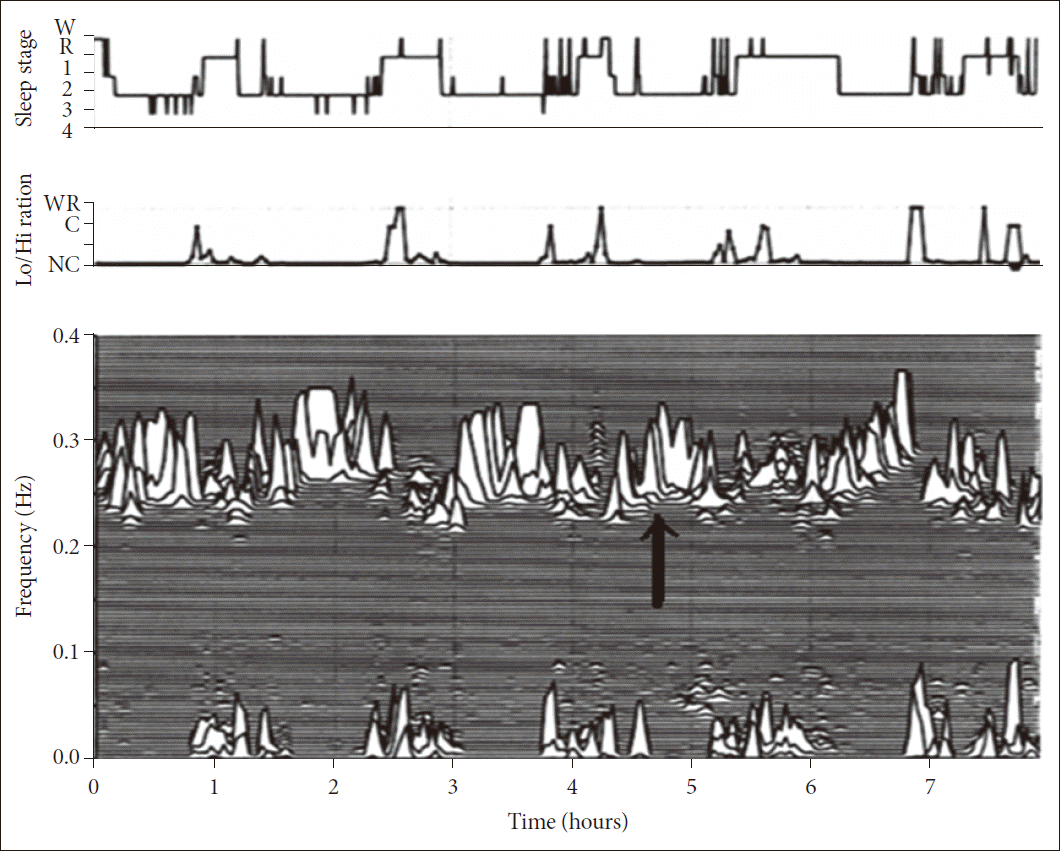
Table 1.Delta spectral power band and sleep spectrograms with polysomnography data
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||