AbstractBackground and ObjectiveObstructive sleep apnea (OSA) is characterized by repetitive episodes of complete or partial upper airway obstruction during sleep. Although, optimal treatment of OSA with continuous positive airway pressure (CPAP) reduce the number of respiratory events during sleep, and thus improve daytime sleepiness, quality of life, and cardiovascular risk, there are few studies that address the long term effects of CPAP treatment. This study aims to determine the long term effects of positive airway pressure (PAP) in compliant OSA patients.
MethodsForty OSA patients naïve to PAP treatment were included. Polysomnography for apnea-hypopnea index, sleep structure, and desaturation index, blood pressure, Epworth Sleepiness Scale (ESS) for excessive daytime sleepiness, and Beck Depression Inventory (BDI) for depressive mood were initially administered before the PAP treatment and after the long term PAP treatment. We have excluded immediate pneumatic effects of the PAP treatment, by administrating a follow up study after 7 days of PAP treatment withdrawal.
ResultsA total of 40 (male: 92.5%, mean age: 54.4 ┬▒ 10.4 yr) patients were enrolled. The duration of PAP treatment was 3.4 ┬▒ 1.4 years. The initial apnea-hypopnea index (AHI) was 50.8 ┬▒ 22.6/hr which decreased to 34.4 ┬▒ 19.1/hr (p Ōēż 0.001). These significant reductions in AHI were seen without Body Mass Index changes (p = 0.707). The sleep architecture revealed no significant changes between the initial and follow up study. The systolic and diastolic blood pressure (DBP) decreased significantly after the long term CPAP treatment (systolic blood pressure pre: 138.5 ┬▒ 18.1, post: 122.3 ┬▒ 13.7, p Ōēż 0.001, DBP pre: 88.7 ┬▒ 13.6, post: 78.5 ┬▒ 11.1, p Ōēż 0.001). ESS and BDI decreased significantly after years of PAP treatment compared to the baseline study (ESS pre: 11.5 ┬▒ 5.3, post: 8.3 ┬▒ 4.7, p Ōēż 0.001, BDI pre: 12.7 ┬▒ 11.6, post: 6.3 ┬▒ 7.0, p = 0.019).
INTRODUCTIONObstructive sleep apnea (OSA) is characterized by repetitive episodes of complete or partial upper airway obstruction occurring during sleep. Excessive daytime sleepiness (EDS), the major presenting complaint, is associated with an increased risk of traffic accidents.1,2 It also impairs quality of life by increasing the risk of hypertension (HTN) and cardiovascular disease.3ŌĆō7 OSA can be treated with continuous positive airway pressure (CPAP). The CPAP working as a pneumatic splint to relieve airway obstruction during sleep eliminates respiratory events.8,9 Reduction of respiratory events during sleep improves daytime sleepiness, quality of life, and cardiovascular risk in randomized-controlled trials.1,10
Previously, baseline and follow up sleep studies of CPAP treatment revealed significant improvement of the apnea-hypopnea index (AHI) index even after the discontinuation of treatment.11ŌĆō13 However, the follow up studies done in these studies were only after one night of treatment withdrawal, and the duration of CPAP treatment was less than 1 year (few months to 1 year). Further, there is only one randomized controlled study investigating the CPAP withdrawal on OSA severity, sleepiness, psychomotor performance, and measures of cardiovascular risk.14
This study investigated the long term effects of OSA after compliant positive airway pressure (PAP)(CPAP, or AutoPAP) therapy. The included subjects are naïve to CPAP treatment with good compliance defined by more the 4 hours use per night. The authors studied the baseline and follow up measurements of severity of OSA syndrome, sleep architectures, subjective daytime sleepiness, cardiovascular risk factors, and mood changes.
METHODSStudy PopulationThe patients were recruited from June 2006 to January 2009 in a Sleep Clinic at the Samsung Medical Center, Korea. All patients were diagnosed with moderate to severe OSA by the initial overnight polysomnography (PSG)(AHI > 15/hr) and were naïve to CPAP treatment. All the patients underwent complete medical examination, and were screened for underlying HTN, diabetes, dyslipidemia, atrial fibrillation, heart failure, stroke and any other disabling medical condition. Body weight, height, and body mass index (BMI) were calculated. We excluded those with localized anatomical defects of the upper respiratory pathway, or with neurological and endocrine disease known to be associated with OSA. We also excluded patients taking drugs active onthe central nervous system and those who suffered from chronic alcoholism. All patients, after diagnosis of OSA were treated with PAP either by CPAP or AutoPAP. Finally, forty compliant PAP users were able to have the follow PSG study. The compliant user indicating averages of at least four hours of use per night were ultimately included.
These patients went through initial studies of overnight PSG, blood pressure, Epworth Sleepiness Scale (ESS), and Beck Depression Inventory (BDI) measurements. The same studies were repeated for a follow up study after years of PAP treatment, however to avoid the immediate effects of PAP treatment, patients were withdrawn from the treatment for 1 week prior to the follow up study.
The overnight PSG was conducted including a 6 channel electroencephalogram (EEG)(F4-M1, F3-M2, C3-M2, C4-M1, O1-M2, O2-M1), a 2 channel electrooculogram, a 4 channel electromyogram for the chin, intercostals muscles, right and left anterior tibialis, and electrocardiogram (ECG)(Embla Co., Broomfield, CO, USA). The EEG and ECG signals were digitized at a sampling rate of 200 Hz. Nasal pressure, plethysmography, thoracic and abdominal breathing movement were also recorded. All PSG studies were manually sleep-staged in 30 second epochs by experienced sleep technologists according to the American Academy of Sleep Medicine (AASM) criteria.15 Respiratory events including obstructive apnea and hypopnea were scored manually based on the AASM criteria. The obstructive sleep apnea syndrome (OSAS) was defined when the AHI was more than 5/hr. Nine patients underwent full-night PSG and 31 patients underwent split-night PSG. Split night PSG is a sleep study during which the first two or more hours of the sleep period are used to assess OSA, and if criteria are met, CPAP titration is performed during the remainder of the night. Criteria for switch overto the CPAP titration is when the AHI > 10/hr. The same procedure was done for the follow up PSG study and of them 7 patients underwent split night PSG and 33 patients underwent full night PSG. The follow-up PSG study was done after CPAP treatment was interrupted for 7 days.
Questionnaires for EDS and depressive mood were done at the initial evaluation of PSG, and at follow up. The severity of EDS was evaluated by using the ESS questionnaire.16 The ESS questionnaire requires subjects to rate the likelihood that they might doze off or fall asleep in eight different everyday situations. The sleepiness is scored from 0 to 3 points, where 3 represent a high likelihood of dozing or falling asleep. BDI contains 21 groups of four statements regarding cognitive, biologic and emotional symptoms of depression, each scored between 0 and 3.17 The ESS scores high when the subject reports sleepiness and BDI scores high when the subject reports a depressive mood. A BDI score of 10 or more indicates mild depressive mood, and 16 or more suggests depression.
Blood pressure was documented in all the enrolled patients regardless of previously diagnosed HTN. Patients were asked to abstain from caffeine containing products, and remain in a stable resting state before the systolic blood pressure (SBP), and diastolic blood pressure (DBP) measurement. Blood pressures were measured in the sitting position during the outpatient clinic visits, baseline during the initial visit and follow up during the last visit before the second PSG study.
Statistical AnalysisContinuous data are expressed as mean (┬▒ standard deviation) or median and categorical data are presented as percent frequencies and percentages. The difference in the pre-PAP treatment and post-PAP treatment values were compared. The variables were compared by paired t-test before and after CPAP therapy. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTSForty OSA patients successfully used PAP for mean 3.4 ┬▒ 1.4 years. The mean age was 54.4 ┬▒ 10.4 years, and there was a significant male predominance (n = 37, 92.5%). The baseline characteristics of the subjects studied are described in Table 1. The mean BMI index was 26.1 ┬▒ 2.7 kg/m3. The mean ESS score was 11.5 ┬▒ 5.3. Among 40 enrolled patients, 10 patients were diagnosed with HTN, 1 with diabetes mellitus, 6 dyslipidemia, 1 ischemic stroke, 3 ischemic heart diseases, 3 atrial fibrillation, 2 valvular heart diseases and none of the patients had a history of congestive heart failure.
All the enrolled patients underwent baseline and follow up sleep studies. The initial baseline PSG results of 40 patients revealed mean AHI of 50.8 ┬▒ 22.6/hr (min: 16.9/hr, max: 118.3/hr). The mean lowest nocturnal desaturation levels were 78.6 ┬▒ 9.30% (min: 61%, max: 89%), which demonstrated that the majority of patients were in the moderate or severe OSA range. After the initial sleep study, 16 patients were treated with CPAP and 24 patients were treated with AutoPAP. We confirmed from the downloaded data regarding the PAP device during the follow up period that these patients were treated with optimal pressure and duration, suggested by mean AHI < 10/hr, and mean time used per day > 4/hr.
The average duration of the PAP uses since the diagnosis were 3.4 ┬▒ 1.4 years. It is remarkable that there was no significant change in BMI (pre: 26.1 ┬▒ 2.7, post: 26.1 ┬▒ 2.7, p = 0.91) during the course of follow up (Table 1, Fig. 1).
Among 40 enrolled patients, there were 7 patients who went through paired full night PSG initially and at follow up. Sleep structures were compared among these 7 patients. The sleep architecture revealed no significant changes between the initial and follow up PSG (Table 2). The AHI reduction was also seen among 7 selected patients (pre-PAP AHI = 45.0 ┬▒ 7.1/hr, post-PAP AHI = 27.0 ┬▒ 15.5/hr, p = 0.014). The significant changes between pre- and post-PAP PSGs in sleep stage I, rapid eye movement (REM) sleep, slow wave sleep (SWS), sleep latency, sleep efficiency, lowest desaturation and arousal index were not seen. The changes between the pre- and post-PAP PSGs were time in bed (TIB)(pre: 320.3 ┬▒ 94.1, post: 421.2 ┬▒ 55.2 p = 0.044), and decrease in wake after sleep onset (WASO)(pre: 32.3 ┬▒ 21.6, post: 15.4 ┬▒ 10.6, p = 0.018).
All the patients administered the PAP treatment: minimum of 4 hours per night were required for the enrollment. The average PAP per day was 356.0 ┬▒ 68.0 minutes, and the rate of days of use were 92.8 ┬▒ 19.0 percent. The faithful PAP treatment of these patients was supported by the findings that during the PAP use the mean pressure was 9.7 ┬▒ 1.7 (min: 6.9, max: 13.5), and mean AHI was 4.2 ┬▒ 3.4 (min: 1.4, max: 22.4). The type of mask used was chosen based on the patientsŌĆÖ preference. 30 used a nasal mask, 9 used nasal pillows, and one used a full face mask.
A significant improvement in AHI was seen compared to the initial AHI, as illustrated in Fig. 1. The average baseline AHI was 50.8 ┬▒ 22.6/hr, which decreased to 34.4 ┬▒ 19.1/hr (p Ōēż 0.001). Among them 5 had an AHI below 15/hr, and none below 5/hr: the majority still remained in a range of moderate to severe OSA. Total of 12 (30.0%) patients had more than a 50% reduction from baseline, 21 (52.5%) patients had < 50% reduction from baseline and 7 (17.5%) patients had an increase in AHI compared to baseline despite optimal use. Each sub-group had no significant BMI between baseline and follow up studies. The exposure to hypoxemia at night improved not only due to a decrease in the frequency of obstructive events but also to an improvement in the mean nocturnal desaturation. The lowest nocturnal desaturation revealed significant change from 78.0 ┬▒ 9.0% before PAP treatment to 82.3 ┬▒ 5.3% after PAP treatment.
After PAP treatment, SBP and DBP significantly decreased by ŌłÆ16.2 and ŌłÆ10.2 mm Hg, respectively (SBP pre: 138.5 ┬▒ 18.1, post: 122.3 ┬▒ 13.7, p Ōēż 0.001, DBP pre: 88.7 ┬▒ 13.6, post: 78.5 ┬▒ 11.1, p Ōēż 0.001)(Table 1, Fig. 1). Subgroup analysis revealed that 10 patients who were diagnosed with HTN and were on anti-hypertensive medication had statistically significant SBP reduction (pre: 140.4 ┬▒ 17.6, post: 126.4 ┬▒ 10.7, p = 0.008), but no significant changes in DBP (p = 0.114). The 30 normotensive patients had statistically significant SBP, and DBP reduction (SBP pre: 138.0 ┬▒ 18.2, post: 120.8 ┬▒ 14.3, p < 0.001, DBP pre: 87.9 ┬▒ 13.3, post: 76.5 ┬▒ 11.6, p < 0.001)
The ESS indicating subjective daytime sleepiness also decreased (pre: 11.5 ┬▒ 5.3, post: 8.3 ┬▒ 4.7, p value < 0.001)(Table 1, Fig. 1). The BDI score significantly decreased after PAP treatment (pre: 12.7 ┬▒ 11.6, post: 6.3 ┬▒ 7.0, p value = 0.019)(Table 1). The initial BDI score revealed that the prevalence of undetected mild depression (BDI score 10ŌĆō15) was 5% at base line and 10% after PAP treatment, and depression (BDI score Ōēź 16) at baseline was 50% and 10% after PAP treatment (Table 1).
DISCUSSIONThis study investigated the long term average use (3.5 years) effects of PAP in compliant OSA patients. We evaluated the severity of OSA, sleep architectures, subjective daytime sleepiness, blood pressure, and depressive mood by BDI score. Similar studies have been reported the change of pre- and post-CPAP treatment; however these reports were focused on the immediate changes caused by withdrawal of nasal CPAP after only a few months of use. This study explored the long term effects of compliant PAP treatment compared to baseline data. Furthermore, to exclude residual PAP effects, follow-up studies were done after PAP treatment withdrawal for 1 week.
The baseline and follow-up PSG indicated a statistically significant decrease of AHI after PAP treatment. Similar studies have been previously reported which indicated that OSA patients, optimally treated for months, show improvement of AHI severity even after the withdrawal of CPAP treatment,11ŌĆō13 These studies were designed in patients with CPAP treatment less than 1 year (a few months to 1 year), and follow-up PSG studies done only after one night of PAP treatment withdrawal. Since these studies have relatively short PAP treatment duration and only one night of treatment withdrawal, it is not clear whether it was a long term effect or an immediate pneumatic effect of the CPAP treatment for AHI reduction. Only one study focuses on the CPAP withdrawal for 2 weeks in OSA patients who have already been on optimal treatment for at least 12 months. The rapid return of OSA on the first night off CPAP was seen, with further changes until the first week of trial, and not much after a week.14 This supports that CPAP withdrawal for 7 days, as in this study, is sufficient enough to represent changes in OSA severity of the subjects after long term treatment.
The strength of this study is that the enrolled patient population was na├»ve to PAP treatment initially and had an average of 3.5 years of PAP use, much longer than the previous studies and withdrawal of PAP treatment was for 7 days. The results of follow-up studies indicate that a long term PAP treatment may have an enlarging effect of the airway or reduce the progression of OSA. There is evidence that OSA worsens over time when left untreated.18,19 Further on, this study had excluded the effect of anatomical changes caused by weight gain or loss during the follow-up period. Thus, relatively independent effects of long term PAP use were seen. The CPAP treatment can improve AHI and may halt and reverse the progression of the underlying pathophysiology of OSA. It provides a physical ŌĆ£pneumatic splintŌĆØ to relieve upper airway obstruction during sleep, thus eliminating respiratory events.13 Further contribution of CPAP treatment has been proposed by the partial reversibility of sensory impairment, due to neuropathy. Previously, it has been proposed that the upper airway neuropathy in OSA is due to the mechanical trauma associated with snoring and apneas, oxidative stress related to hypoxia-oxygenation, and inflammation resulting from both of these insults.9,13,14,20ŌĆō22 The partial improvement of OSA, can in part be explained by the reversibility of such a mechanism after long term treatment with PAP. Furthermore, increased intra-airway pressure during PAP treatment for several years may enlarge the airway opening although we did not follow-up the airway magnetic resonance imaging study.
The sleep structure of patients with OSAS is characterized by the loss of physiological REM/NREM alternation as well as by a deficit of REM and SWS and excess of sleep stages 1 and 2.23ŌĆō25 This can be caused by sleep fragmentation as a result of the high amount of arousals related to respiratory events.23 The disturbance of sleep structures have been reported to improve with CPAP treatment.12,23 However, long term PAP did not improve the disturbed sleep structure that was seen at the initial sleep study. However, only 7 patients had both pre- and post-PAP full night PSG and post-PAP AHI was still abnormal. This result correlates well with previous studies of sleep structure changes after CPAP withdrawal for 1 and 14 days.12ŌĆō14 However, WASO decreased after long term PAP treatment in the present study.
Cardiovascular risk factor assessments by changes in blood pressure revealed a significant decrease in SBP and DBP after PAP treatment. In our study, compared to the pre-PAP period, SBP and DBP measured at post-PAP treatment decreased significantly by 16.2 and 10.2 mm Hg, respectively. Remarkably, the reductions of blood pressure were also seen in the normotensive patients. There are numerous reports on the effects of CPAP on the reduction of BP in patients with OSA and sustained HTN. The decrease of blood pressure after 1 or 2 months of CPAP treatment varies from 2.5 to 8 mm Hg depending on the AHI severity, and initial blood pressure.26 Furthermore, significant increase in systolic (+ 8.5 mm Hg) and diastolic (+ 6.9 mm Hg) blood pressure after 2 week of CPAP treatment withdrawal have been reported.14 However, this study was focused on the changes of blood pressure between the optimal CPAP treatment period and after CPAP withdrawal. Our results, which compared blood pressures between pre- and post-PAP treatment periods in long term compliant users, indicated a significant decrease in blood pressure after PAP treatment. Although, rapid returns of blood pressure after CPAP withdrawal are also reported, years of effective PAP use and AHI decrease post-PAP treatment may have contributed to the stable reduction of the baseline blood pressure.
The EES scores indicating subjective daytime sleepiness in this study have decreased from an average of 11.5 to 8.3. This is contrary to previous studies, which indicated a rapid increase in ESS scores after CPAP treatment withdrawal.11,12 However, these studies represent only 1 night of treatment withdrawal, in patients who have been on treatment for a few months to l year. Furthermore, sudden withdrawal might have induced rebound sleepiness.
The BDI has been used to assess depressive mood.27 The BDI scores are reported to be high in 20ŌĆō25% of OSA patients.28 How-ever, the results of studies on BDI changes following CPAP treatment were controversial. Some studies have reported a decrease in BDI score after CPAP treatment29,30 whereas others have not.31,32 Our results indicated a significant decrease of BDI score after 3.5 years of PAP treatment and one week withdrawal of PAP treatment.
Our study is not without limitations: First, this study does not have a sub-therapeutic or placebo group for comparison. We excluded partially treated or inadequately used patients by enrolling patients who are compliant PAP users. Second, BDI, and ESS scores are based on the patients subjective measurements, other confounding factors should be considered, and straightforward association requires cautious interpretation. Especially, BDI can be scoredhigher with other related factors such as fatigue, loss of interest, decreased libido, and poor concentration, which have not been evaluated in our study. Third, although we have compared PSG parameters between baseline, and follow up studies, cautious interpretation is needed because follow up full night or split night PSG were selected regardless of which were done for the baseline study.
This study confirmed that after years of faithful PAP treatment for OSA, one week of PAP treatment withdrawal demonstrated a significant decrease of AHI. Moreover, long term PAP treatment induced the improvement in blood pressure, daytime sleepiness, and depressive mood, even after the withdrawal of treatment.
REFERENCES1. Siccoli MM, Pepperell JC, Kohler M, Craig SE, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep 2008;31:1551-8.
2. George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001;56:508-12.
3. Gottlieb DJ, Yenokyan G, Newman AB, OŌĆÖConnor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010;122:352-60.
4. Redline S, Yenokyan G, Gottlieb DJ, Shahar E, OŌĆÖConnor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182:269-77.
5. Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med 2007;167:757-64.
6. Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 2010;7:677-85.
7. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53.
8. Strohl KP, Redline S. Nasal CPAP therapy, upper airway muscle activation, and obstructive sleep apnea. Am Rev Respir Dis 1986;134:555-58.
9. Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1:862-5.
10. Kohler M, Pepperell JC, Casadei B, Craig S, Crosthwaite N, Stradling JR, et al. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J 2008;32:1488-96.
11. Kribbs NB, Pack AI, Kline LR, Getsy JE, Schuett JS, Henry JN, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147:1162-8.
12. Sforza E, Lugaresi E. Daytime sleepiness and nasal continuous positive airway pressure therapy in obstructive sleep apnea syndrome patients: effects of chronic treatment and 1-night therapy withdrawal. Sleep 1995;18:195-201.
13. Leech JA, Onal E, Lopata M. Nasal CPAP continues to improve sleep- disordered breathing and daytime oxygenation over long-term follow-up of occlusive sleep apnea syndrome. Chest 1992;102:1651-5.
14. Kohler M, Stoewhas AC, Ayers L, Senn O, Bloch KE, Russi EW, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 2011;184:1192-9.
15. Iber C. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2007.
16. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5.
17. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71.
18. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000;283:1829-36.
19. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378-84.
20. Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 1991;144(3 Pt 1):494-8.
21. Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis 1993;148:462-6.
22. Bergeron C, Kimoff J, Hamid Q. Obstructive sleep apnea syndrome and inflammation. J Allergy Clin Immunol 2005;116:1393-6.
23. Fietze I, Quispe-Bravo S, H├żnsch T, R├Čttig J, Baumann G, Witt C. Arousals and sleep stages in patients with obstructive sleep apnoea syndrome: Changes under nCPAP treatment. J Sleep Res 1997;6:128-33.
24. Guilleminault C. Clinical features and evaluation of obstructive sleep apnea. In Kryger MH, Roth T, Dement WC (eds.), Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier-Saunders; 1989. pp. 552-9.
25. McNamara SG, Cistulli PA, Strohl KP, Sullivan CE. Clinical aspects of sleep apnea. In Saunders NA, Sullican CE (eds.), Sleep and breathing. 2nd ed. New York: Marcel Dekker; 1994. pp. 493-528.
26. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009;373:82-93.
27. Beck AT, Rush JA, Shaw BF, Emery G. Cognitive therapy of depression. New York, NY: Guilford Press; 1979.
28. Watson R, Greenberg G, Bakos L. Sleep apnea and depression. Sleep Res 1987;16:293.
29. Means MK, Lichstein KL, Edinger JD, Taylor DJ, Durrence HH, Husain AM, et al. Changes in depressive symptoms after continuous positive airway pressure treatment for obstructive sleep apnea. Sleep Breath 2003;7:31-42.
30. Klonoff H, Fleetham J, Taylor DR, Clark C. Treatment outcome of obstructive sleep apnea. Physiological and neuropsychological concomitants. J Nerv Ment Dis 1987;175:208-12.
Fig.┬Ā1.Comparative illustration of changes between initial and follow up studies. A: Apnea hypopnea index. B: Body Mass Index. C: Epworth Sleepiness Scale. D: Systolic blood pressure. *Statistically significant changes. 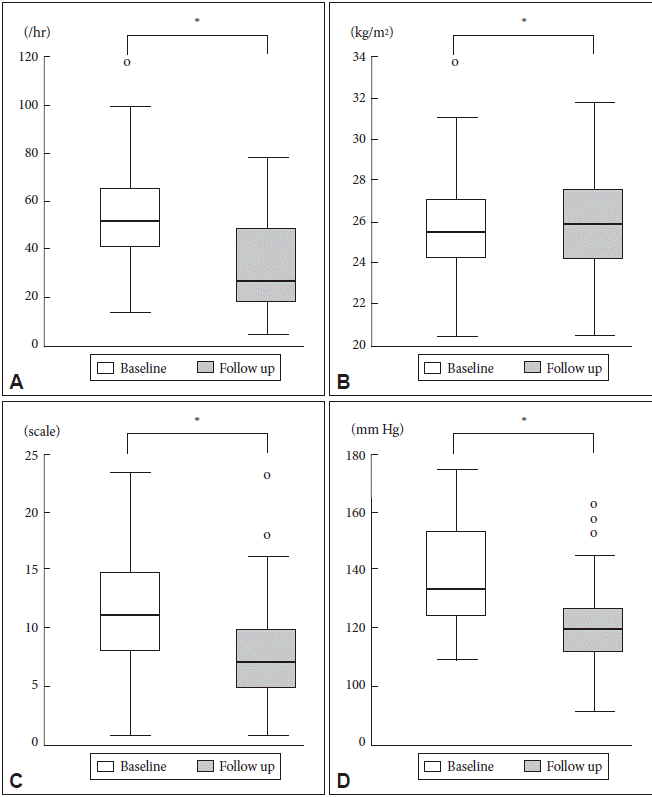
Table┬Ā1.Clinical characteristics, sleep parameters, sleepiness, and depressive mood of enrolled patients Table┬Ā2.Sleep parameters in patients with full night PSG PSG: polysomnography, CPAP: continuous positive airway pressure, SD: standard deviation, TST: total sleep time, TIB: time in bed, WASO: wake after sleep onset, REM: rapid eye movement, AHI: apnea hypopnea index, NREM: non rapid eye movement, SWS: slow wave sleep, PLMS: periodic limb movements in syndrome, MAI: movement arousal index. |
|
|||||||||||||||||||||||||||||||||||||