INTRODUCTION
Obstructive sleep apnea (OSA) is a very common disease. It occurs in about 2%–4% of the total population worldwide [1] and 3.2%–4.5% of the population in Korea. OSA is becoming a common disease as members of the modern society are aging and obese as a whole [2]. OSA is associated with diseases such as hypertension, diabetes, heart disease, stroke, depression, erectile dysfunction, and cognitive impairment. It causes symptoms such as daytime sleepiness, decreased productivity, and decreased quality of life. It also causes anxiety and insomnia in sleep partners, creating social problems [3-5].
The most useful and accurate test for diagnosing OSA is the standard polysomnography (PSG). Since it is difficult to diagnose sleep apnea only with clinical symptoms, PSG is recognized as a relatively objective and reliable test to check for symptoms, predisposition, and risk factors of OSA [6]. However, the standard PSG is complicated and the test is conducted in a laboratory with a sleep environment different from home, which can show a different sleep pattern than usual. In addition, it has disadvantages of being expensive and needing manpower [7,8].
As the association between OSA and cardiovascular disease is known with the development of treatment method, the demand for PSG for diagnosis and treatment of OSA has increased rapidly. However, after the onset of COVID-19, it became difficult to conduct standard PSG because of fear of visiting hospitals and the reluctance to use the same laboratory as others. Since screening tests for patients with OSA symptoms clinically cost less, interest in mobile sleep tests showing high accuracy has increased.
PSG can be divided into four types. Type 1 is a full attended PSG, which tests through more than 7 channels that are essential under the monitoring of sleep-related medical personnel in a sleep laboratory. Type 2 is full unattended PSG. With this type, inspectors do not have to monitor during sleep inspection time, but perform inspections through 7 or more channels [9]. Type 3 is a test with 4 to 7 limited channels. Type 4 is a test with 1 to 2 channels including the normal oxygen saturation channel [9].
Our hospital sleep clinic is using ApneaLinkTM (ResMed, San Diego, CA, USA), a portable sleep device, to perform screening tests for patients suspected of having obstructive apnea due to snoring or sleep apnea or refusing standard PSG. ApneaLinkTM is a portable sleep device that receives information through five channels to determine the degree of OSA. This study attempted to analyze the correlation between ApneaLinkTM (a mobile sleep test device used for checking the pattern of breathing during sleep) and PSG and to evaluate the usefulness of ApneaLinkTM as a screening test for OSA.
METHODS
Subjects
This study targeted patients who visited our hospital sleep clinic with suggestive OSA symptoms and underwent PSG and ApneaLinkTM from January 2018 to December 2020. When conducting ApneaLinkTM, sleeping with the TV turned on or sleeping in a noisy environment, which could interfere with normal sleep, was prohibited due to environmental similarity to the PSG laboratory. Patients with nasal congestion or respiratory diseases were excluded from this study. A total of 166 patients were selected. Their medical records were retrospectively analyzed. This study was approved by the Institutional Review Board (IRB) of Dong-A University Hospital (approval number: DAUHIRB-22-125).
Polysomnography Data
All patients underwent full type 1 PSG. Among items recorded, Apnea-Hypopnea Index (AHI) and Respiratory Disturbance Index (RDI) were analyzed. Apnea was defined as a case in which more than 90% of the airflow was lost for more than 10 seconds. Hypopnea was defined as a decrease of more than 50% as oxygen saturation dropped by more than 3% for more than 10 seconds. AHI was defined as mean apnea and hypopnea per hour. RDI was defined as the average number of respiratory disorders per hour (obstructive apnea, hypopnea, and awakening-related respiratory events).
Screening Test with ApneaLinkTM
The ApneaLinkTM mechanical device has a button to press at the start and end, a lamp that emits light according to the rhythm of breathing during sleep, a sensor that connects a cannula which measures pressure in the nasal cavity, and a cable that can send stored data to a computer. It is attached to the patient’s chest using a belt. The nasal probe is connected into the nose and fixed. After that, the patient or patient’s sleeping partner was trained to press the start button before falling asleep and the stop button when waking up. Respiratory effort, pulse, oxygen saturation, intranasal airflow, and snoring were recorded for up to five channels. Results were automatically analyzed (automated scoring) and stored in ApneaLinkTM.
In ApneaLinkTM, if more than 90% of airflow decreased for at least 10 seconds, it was recorded as apnea. If more than 50% of airflow decreased for at least 10 seconds, it was automatically recorded as hypopnea. AHI index was then calculated. In ApneaLinkTM, the Risk Indicator (RI) Index is a numerical value that corresponds to RDI in a PSG. RI was calculated based on the number of apnea, hypoventilation events, and total airflow obstruction during respiration (RI = AHI + 10 × [0.8 × FL + 1.2 × FS] / If, FL = flow limited breaths without snoring, FS = flow limited breaths with snoring, If = total number of breaths).
Statistical Analysis
Patients were classified into two groups according to AHI and RDI. In each group, according to AHI and RDI values, those with 5 or more but less than 15, those with 15 or more but less than 30, and those with 30 or more were classified as mild, moderate, and severe sleep apnea, respectively. Pearson correlation was used to evaluate correlations of AHI and RDI in PSG with AHI and RI in ApneaLinkTM. SPSS 24.0 (IBM Co., Armonk, NY, USA) was used as the analysis program. Statistical significance was considered when p-value was less than 0.05.
In addition, in order to confirm its usefulness as a screening test for patients with moderate or more severe sleep apnea, we tried to set cut-off values for AHI and RI of ApneaLinkTM using a receiver operating characteristic (ROC) curve. At this time, discriminating variables were set to be AHI and RI values of ApneaLinkTM. The ROC curve was analyzed with discrimination criteria (0 when the AHI was less than 15 and 1 when the AHI was 15 or more). Similarly, when RDI was less than 15, it was set to be 0. When it was more than 15, it was set as 1. The ROC curve was then analyzed.
RESULTS
Of a total of 166 patients, 128 (77.1%) were males and 38 (22.9%) were females. The mean age of patients was 52.44 ± 14.55 years (range, 20–84 years). The mean BMI was 26.65 ± 4.43 kg/m2.
When analyzing correlations of AHI and RDI of PSG with AHI and RI of ApneaLinkTM, correlation coefficients between AHI or RI of ApneaLinkTM and AHI of PSG were both 0.647, showing a positive correlation (p < 0.001). Correlation coefficients between AHI or RI of ApneaLinkTM and RDI of PSG were both 0.637, showing a positive correlation as well (p < 0.001) (Fig. 1).
According to AHI and RDI, subjects were divided into mild (5–15), moderate (15–30), and severe (≥ 30) sleep apnea groups. When average values of AHI and RI of ApneaLinkTM of groups were compared, they were significantly different among groups (p < 0.001). Average values of AHI and RI of each group were within the range of corresponding group values of the first divided criterion (Table 1).
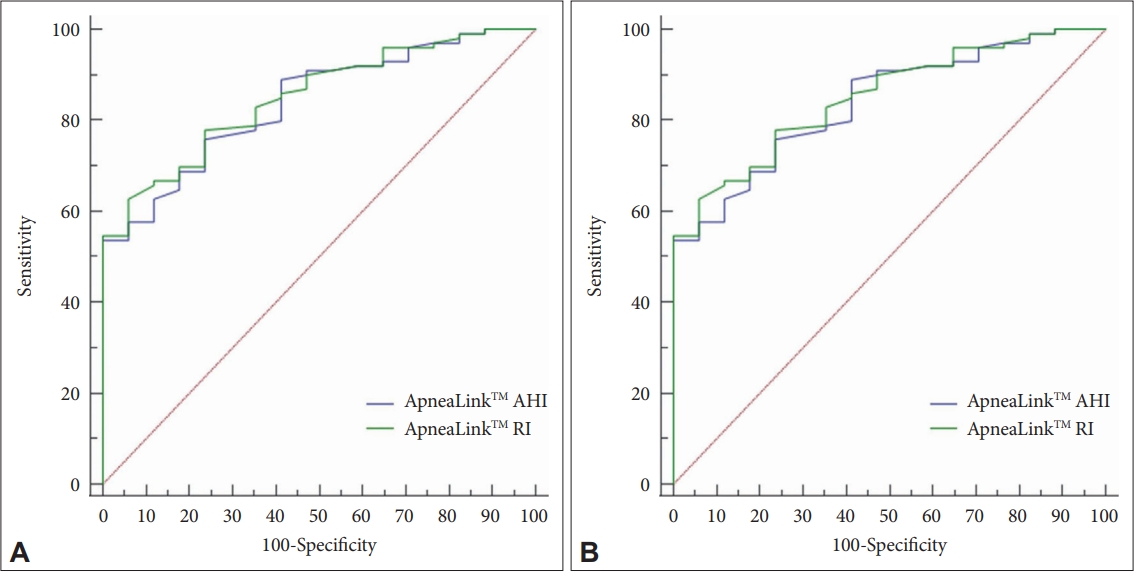
When AHI of PSG was analyzed using ROC curve to select moderate or severe sleep apnea patients, a cut-off value of AHI at 32.1 in ApneaLinkTM had a sensitivity of 53.54%, a specificity of 100%, and an area under the curve (AUC) of 0.838. A cut-off value of RI at 29.0 in ApneaLinkTM had a sensitivity of 62.63%, a specificity of 94.12%, and an AUC of 0.849 (Table 2 and Fig. 2A).
When the RDI of PSG was analyzed with ROC curve, a cut-off value of 19.0 for AHI of ApneaLinkTM showed a sensitivity of 76.29%, a specificity of 78.95%, and an AUC of 0.846. Also, a cut-off value of 29.0 for RI of ApneaLinkTM had a sensitivity of 62.89%, a specificity of 94.74%, and an AUC of 0.856 (Table 2 and Fig. 2B).
DISCUSSION
OSA is a very common disease observed in 4% of men and 2% of women over the age of 30 years [10]. It is known that PSG is the gold standard for determining the severity of sleep apnea and detecting functional abnormalities [6,11]. However, the standard PSG is costly. The number of equipment is limited compared to the number of patients. Therefore, interest in portable PSG, which can replace standard PSG at a low cost is rapidly increasing. Many studies have been conducted. According to a study by Littner [12], when the clinical aspect and oxygen saturation measurement were combined, only 32.4% of patients were classified as OSA. However, when the upper airway and body measurements were used, sensitivity and specificity of OSA diagnosis were 97.6% and 100%, respectively, with positive and negative predictive values being very high at 100% and 88.5%, respectively. Pang et al. [13] have reported a high correlation of 0.87 (p < 0.001) in RDI as a result of comparing and analyzing WatchPAT, a type of mobile sleep test, and standard PSG in 30 patients. Pillar et al. [14] have reported that the correlation coefficient between Sleepstrip, a mobile sleep tester that measures air flow, and AHI of standard PSG is 0.71, showing a significant correlation. In this study, correlations of AHI and RDI of PSG with AHI and RI of ApneaLinkTM were analyzed. Correlation coefficient of AHI or RI of ApneaLinkTM with AHI of PSG was both 0.647, which showed a high positive correlation. Between RDI of PSG and AHI or RI of ApneaLinkTM, the correlation coefficient was both 0.637, which showed a similarly high positive correlation (Fig. 1). Also, in a previous study, ApneaLinkTM had a sensitivity of 91% and a specificity of 73%, with a positive predictive value of 0.9 and a negative predictive value of 0.72 [15]. In other words, it can be judged that ApneaLinkTM has high correlation and predictability with PSG.
To confirm the clinical usefulness of ApneaLinkTM in this study, the cut-off value of ApneaLinkTM was obtained using the ROC curve. All four data in Table 2 had an AUC of 0.8 or higher, confirming very good performance. However, the cut-off value of 19.0 for RDI and ApneaLinkTM AHI tests was the closest to the value of 15, which is the actual discriminant value set to discriminate moderate or more severe sleep apnea. In other words, when the ApneaLinkTM AHI test is performed, the sensitivity that can be predicted by the PSG RDI of 15 or higher for those 19 or higher is 76.29% and the specificity is 78.95% (Table 2 and Fig. 2). Therefore, it can be said that ApneaLinkTM is most suitable for use as a screening test for patients with moderate or more severe sleep apnea with RDI of 15 or higher for PSG.
To check breathing patterns during sleep, it is important to accurately record the flow of inhaled and exhaled air. Therefore, devices such as thermistor, nasal pressure, pneumotachometer, and pulse oximetry have been developed and used [16]. Thermistor is a device that can detect the presence or absence using difference in electrical resistance according to temperature difference between inspiration and expiration. It is known to be inaccurate for quantitatively evaluating respiration [17]. Recently, a nasal pressure measuring device has been used to measure air flow, showing a high sensitivity in detecting hypoventilation or respiratory effort related arousal (RERA). It can measure the number of RERA or upper airway resistance. However, it has been reported that the nasal pressure measurement device and the esophageal pressure monitoring device show a statistically significant agreement [18,19]. ApneaLinkTM used in this study uses a nasal pressure measuring device to measure the respiratory index. Thus, it measures the respiratory status more accurately than using a thermistor.
As a result of this study, mobile PSG using ApneaLinkTM is considered to be inexpensive and useful for simple and inexpensive screening for OSA. However, there are some limitations in the use of ApneaLinkTM. First, it may respond too sensitively to the air flow which might appear as apnea during mouth breathing. The total sleep time might be increased by the patient’s caregiver actuating a mechanical device, which may result in lower AHI. Also, OSA cannot be differentiated from central sleep apnea, sleep posture is not reflected, and other sleep disorders that may accompany OSA cannot be detected. For the diagnosis of OSA, in principle, standard PSG should be performed. However, since ApneaLinkTM is easily available, it will be useful in the case of follow-up PSG in patients who have been already diagnosed with OSA, for patients with severe symptoms of OSA who need to start treatment immediately, and for patients who cannot be tested in a sleep laboratory. Therefore, ApneaLinkTM is considered to be useful for OSA screening when it is difficult for patients to visit a hospital directly and perform PSG after the onset of COVID-19.
In conclusion, as a result of this study, the correlation between ApneaLinkTM and PSG was confirmed. Correlation coefficient of AHI or RI of ApneaLinkTM with AHI of PSG was both 0.647, which showed a high positive correlation. Between RDI of PSG and AHI or RI of ApneaLinkTM, the correlation coefficient was both 0.637, which showed a similarly high positive correlation.
In addition, when using ROC curve, a cut-off value of 19.0 showed a sensitivity of 76.29% and a specificity of 78.95%, which can be predicted with a RDI of PSG of 15 or higher. It can be said that the AHI of ApneaLinkTM is most suitable for use as a screening test for patients with moderate or more sever sleep apnea with an RDI of PSG of 15 or higher. These results indicate that ApneaLinkTM is useful for screening for OSA, which is inexpensive and simple.