AbstractBackground and ObjectiveMany studies have indicated that obstructive sleep apnea (OSA) is linked to the development of cancer. However, there have been few studies about the link between OSA and renal cell carcinoma (RCC). This study investigated the relationship between OSA and RCC by analyzing the data from the Korea National Health Insurance Service.
Methods198574 patients (≥ 20 years of age) newly diagnosed with OSA from 2007 to 2014 were included and 992870 control groups were selected through propensity score matching according to sex and age. The average follow-up period was 4.6 years. The primary outcome was newly diagnosed RCC. The hazard ratio for RCC in patients with OSA was compared to that in the control group.
INTRODUCTIONObstructive sleep apnea (OSA) is characterized by repeated upper airway collapse during sleep, which causes frequent respiratory cessation and oxygen desaturation [1]. It affects 4% of men and more than 2% of women. It is a common disorder worldwide and its prevalence has been associated with an increased prevalence of obesity [2]. OSA can lead to a variety of complications, including hypertension, heart failure, stroke, insomnia, depression, dementia, diabetes, and premature death. Recently, evidence supports a potential association between OSA and a variety of cancers, including lymphoma, breast cancer, and prostate cancer [3-5].
Intermittent hypoxia, a key feature of OSA, is suspected to influence the pathogenesis of these cancers through the production of oxygen radicals and hypoxia-inducible transcription factor (HIF) [6]. Furthermore, HIF is also related to occurrence of renal cell carcinoma (RCC) [7]. Therefore, it is presumed that OSA and RCC may also be related. However, there are few reports on the association between the RCC and OSA. Since the occurrence of RCC is uncommon, it is necessary to observe large-scale cohorts for a long time to prove this.
In recent times, the Korea National Health Insurance Service (KNHIS) database has been released for research purposes in Korea. The objective of this study was to evaluate whether the incidence of RCC is higher among patients with OSA than control using the KNHIS database.
METHODSData SourceThe KNHIS is a public health insurance system that covers almost the entire population of Korean [8]. The National Health Insurance is administered by the Korea government and contains long-period data of about 50 million people. KNHIS provides robust data on patient demographics, diagnosis, intervention, and prescribing. The database adopts the 6th edition of the Korean Classification of Diseases, which is a translation of the 10th edition of the International Classification of Diseases. Researchers can use KNHIS data with the approval of local Institutional Review Boards. We also used this data the approval of Konkuk University Hospital Institutional Review Committee (KUMC 2020-03-040).
Study Population and DesignPatients aged more than 20 years with newly diagnosed OSA from 2007 to 2014 were categorized as the OSA group. The control group were not diagnosed with OSA during the same period and matched by age and sex using propensity score. The number of subjects in the control group was five times that in the OSA group. The primary outcome of this study was the incidence of newly diagnosed RCC. Patients diagnosed with any type of cancer prior to enrollment were excluded. The enrollment process was presented in Fig. 1.
Data CollectionThe following baseline data was collected: age, sex, income level (four quintiles), comorbidities including hypertension, dyslipidemia, diabetes, stroke, chronic obstructive pulmonary disease, and ischemic heart disease (Table 1).
Statistical AnalysisA Kaplan-Meier plot without covariance correction was created to show the difference in incidence of RCC between OSA and control groups. The incidence of RCC was calculated by dividing the number of events by the total time at risk. The Cox proportional hazards model was used to determine the relative risk ratio of OSA to the incidence of RCC. Two different models were applied. Model 1 was not adjusted while Model 2 was adjusted for income level, diabetes, hypertension, and dyslipidemia known to be associated with the development of RCC [8-11]. Results are presented as means and 95% confidence intervals (95% CIs). All statistical analyzes were performed using R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTSA sum of 49570064 subjects were included in the KNHIS in 2007 and the number was not changed much for each subsequent year up to 2014. From 2007 to 2014, 198574 patients were newly diagnosed with OSA and 992870 subjects were selected as controls. A demographic data is summarized Table 2.
The Kaplan-Meier Plot between the Obstructive Sleep Apnea Group and ControlsThe Kaplan-Meier plot shows that the incidence of RCC was higher in the OSA group than in the control group (Fig. 2).
The Hazard Ratio of the Obstructive Sleep Apnea Group Compared to That of the Control Group for the Incidence of Renal Cell CarcinomaThe Cox proportional hazards model showed that the hazard ratio in the OSA group for the incidence of RCC was significantly higher for both models. The hazard ratio based on Model 1, which was not adjusted for covariates, was 1.65 (95% CI, 1.41–1.93). The result did not change much after adjusting income level and various comorbidities in Model 2 (Table 3). There was no difference in the hazard ratios by sex and age (Tables 4 and 5).
DISCUSSIONIn this study, we demonstrated that the incidence of RCC was higher in the OSA group. The OSA group showed 1.65 times higher risk of RCC than the non-OSA group after adjusting for age and various comorbidities. There was no difference in the hazard ratio between men and women.
Recent studies have shown the association between OSA and aggressiveness of clear cell RCC and that OSA is associated with a high Fuhrman grade [12]. This study indirectly supports our results that RCC incidence increased in the OSA group. However, KNHIS does not provide data about the cancer stage and pathology, which makes it impossible to reconfirm the association between OSA and aggressiveness of RCC.
Since there have no previous studies about the association between OSA and RCC, the mechanism by which OSA contributes to RCC has not been studied. However, there are a few possible explanations. First, intermittent hypoxia is considered to be a strong risk factor for the occurrence of several cancers [6]. Hypoxic conditions induce the production of HIF-1a, which is involved in angiogenesis and cancer metastasis [13]. HIF-1a is also overexpressed in renal cancer cells, suggesting a link between OSA and RCC [7]. Second, sleep fragmentation is also suspected to be involved in the development of cancer. Sleep fragmentation induces the dysregulation of circadian rhythms, inadequate sleep duration, and suppression of melatonin levels. Several studies have attempted to identify the potential role of melatonin in reducing the risk of kidney cancer [14,15]. As seen in prostate cancer [16,17], melatonin promotes apoptosis of the renal cancer cells and suppresses the progression of RCC [14,15]. These studies imply that the increased risk of RCC in people with OSA might be due to a low level of melatonin.
This study has several limitations. First, OSA was defined using only coding information in KNHIS, and polysomnography results could not be confirmed. Therefore, it was not possible to assess the severity of OSA either. Second, the control group may include undiagnosed OSA patients. In addition, there were no data on lifestyle, exercise, smoking, drinking habits, nutritional status, and body mass index, so risk factors important for cancer were not fully included in the analysis. Also, there was no information about the type and stage of RCC.
Despite all these limitations, this study is the first evidence to show that patients with OSA have a higher incidence of RCC than controls, and further studies are needed in the future.
In conclusion, the incidence of RCC was significantly higher among patients with OSA than among controls. The adjusted hazard ratio was 1.65 (95% CI, 1.41–1.93).
NOTESAvailability of Data and Material
The datasets generated or analyzed during the current study are available in the National Health Insurance Sharing Service repository (https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do).
Author Contributions
Conceptualization: all authors. Data curation: Jae Hoon Cho. Formal analysis: Hyoung Keun Park, Woo Suk Choi. Investigation: Jae Hoon Cho. Methodology: Jae Hoon Cho. Project administration: Hyoung Keun Park, Woo Suk Choi. Resources: Jae Hoon Cho. Software: Jae Hoon Cho. Supervision: Jae Hoon Cho. Validation: Hyoung Keun Park, Woo Suk Choi. Visualization: Jae Hoon Cho. Writing—original draft: Hyoung Keun Park, Woo Suk Choi. Writing—review & editing: Jae Hoon Cho.
REFERENCES1. Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:144-53.
3. Choi JH, Kim SY, Han KD, Cho JH. The incidence of non-Hodgkin lymphoma is increased in patients with obstructive sleep apnea. Leuk Res 2020;98:106455.
4. Choi JH, Lee JY, Han KD, Lim YC, Cho JH. Association between obstructive sleep apnoea and breast cancer: The Korean National Health Insurance Service Data 2007-2014. Sci Rep 2019;9:19044.
5. Lee EJ, Suh JD, Cho JH. The incidence of prostate cancer is increased in patients with obstructive sleep apnea: results from the national insurance claim data 2007-2014. Medicine (Baltimore) 2021;100:e24659.
6. Prabhakar NR, Peng YJ, Nanduri J. Hypoxia-inducible factors and obstructive sleep apnea. J Clin Invest 2020;130:5042-51.
7. Lidgren A, Hedberg Y, Grankvist K, Rasmuson T, Bergh A, Ljungberg B. Hypoxia-inducible factor 1alpha expression in renal cell carcinoma analyzed by tissue microarray. Eur Urol 2006;50:1272-7.
8. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol 2019;75:74-84.
9. Otunctemur A, Ozbek E, Sahin S, Dursun M, Besiroglu H, Koklu I, et al. Diabetes mellitus as a risk factor for high grade renal cell carcinoma. Asian Pac J Cancer Prev 2014;15:3993-6.
10. Zhang Q, Chen P, Tian R, He J, Han Q, Fan L. Metabolic syndrome is an independent risk factor for Fuhrman grade and TNM stage of renal clear cell carcinoma. Int J Gen Med 2022;15:143-50.
11. Hellenthal NJ, Bermejo CE. The role of socioeconomic status in renal cell carcinoma. Urol Oncol 2012;30:89-94.
12. Vilaseca A, Nguyen DP, Vertosick EA, Corradi RB, Musquera M, Pérez M, et al. Obstructive sleep apnea and Fuhrman grade in patients with clear cell renal cell carcinoma treated surgically. World J Urol 2017;35:51-6.
13. Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 1999;59:5830-5.
14. Maleki Dana P, Reiter RJ, Hallajzadeh J, Asemi Z, Mansournia MA, Yousefi B. Melatonin as a potential inhibitor of kidney cancer: a survey of the molecular processes. IUBMB Life 2020;72:2355-65.
15. Wen YC, Lin YW, Chu CY, Yang YC, Yang SF, Liu YF, et al. Melatonin-triggered post-transcriptional and post-translational modifications of ADAMTS1 coordinately retard tumorigenesis and metastasis of renal cell carcinoma. J Pineal Res 2020;69:e12668.
Fig. 2.Kaplan-Meier plot showing the incidence of renal cell carcinoma among patients with OSA. It occurs more frequently in the OSA group than in the control group. OSA, obstructive sleep apnea. 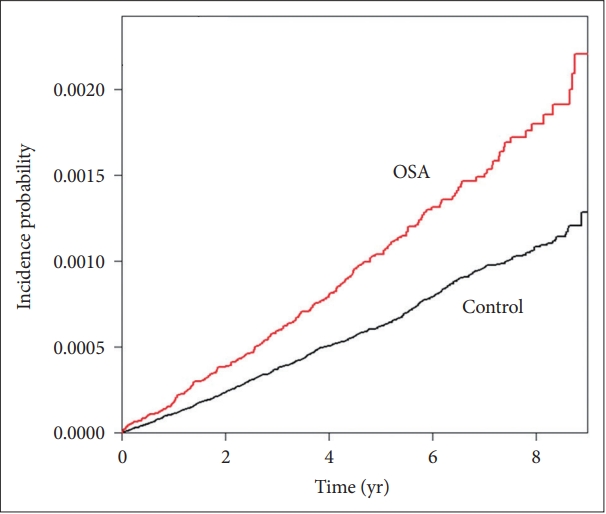
Table 1.Working definitions derived from the insurance claims data Table 2.Demographics of patients in the OSA and control groups Table 3.OSA hazard ratio for the incidence of renal cell carcinoma
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||