INTRODUCTION
Perry syndrome is a rare autosomal-dominant disease characterized by early-onset parkinsonism, weight loss, depression, psychiatric symptoms and central hypoventilation [1,2]. The diagnostic criteria of Perry syndrome are suggested as 1) parkinsonism, family history plus mutation in the exon 2 of dynactin 1 (DCTN1) gene, or 2) parkinsonism, psychiatric symptoms, and respiratory symptoms plus TAR DNA-binding protein (TDP-43) pathology [3]. Herein, we report a 51-year-old man with advanced parkinsonism (the Unified Parkinson’s Disease Rating Scale 63 and Hoehn & Yahr stage 5). 18F-fluorinated N-3-fluropropyl-2β-carboxymetoxy-3β-nortropane positron emission tomography showed a marked loss of dopamine transporters. Findings of DCTN1 mutation supported his diagnosis. The autopsy revealed TDP-43 pathology. As the sleep features of Perry syndrome are mostly unknown, we focus on the polysomnographic (PSG) findings and further discuss the correlations between PSG and neuropathological findings.
CASE REPORT
Polysomnographic Findings
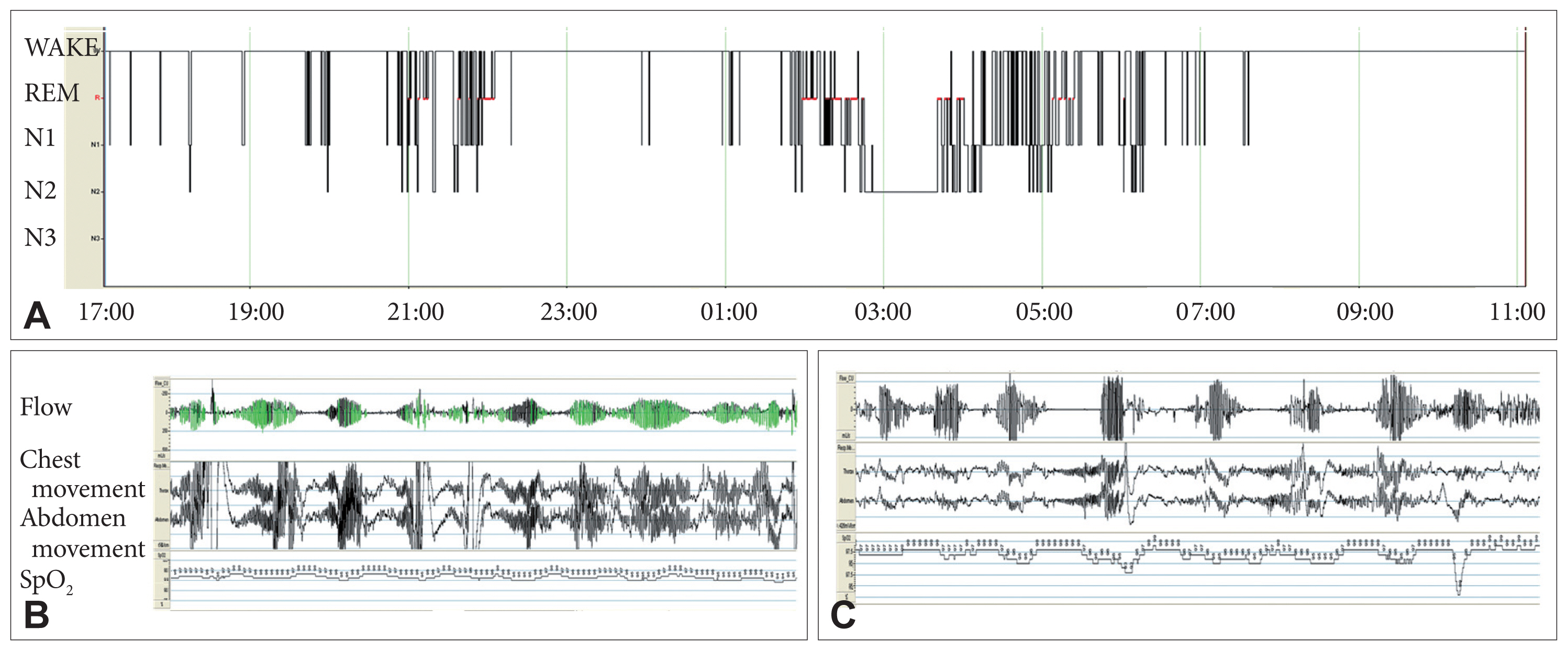
Although extensive interviews were conducted with the patient’s family members, they knew little about his sleep-wake patterns. He was completely bed-ridden when he took the PSG exam. Therefore, we studied the patient’s sleep patterns using PSG from 17:00 to the next day 11:00 (Fig. 1).
While waking, an arterial blood gas analysis indicated mild hypercapnia without hypoxemia (pH, 7.42, Pco2, 51 mm Hg, HCO3−, 32.5 mm Hg, Po2, 91.7 mm Hg). Cheyne-Stokes respiration (CSR), the periodic breathing pattern in which the tidal volume gradually waxes and wanes in a crescendo-decrescendo manner, was evident. He slept 248.5 min, and two distinct periods of sleep, from 21:00 to 22:00 and from 02:00 to 06:00, were identified. The proportion of N1 was elevated to 41.6% (normal range of 1–5%) and N2 was reduced to 22.2% (normal range of 40–50%). Deep sleep was absent. The proportion of REM was 26.2% which was in the normal range. While sleeping, the patient experienced semi-periodic breathing, although the breathing was discrepant from CSR. He had apneas with obstructive apnea index of 11.4/h, hypopnea index of 33.6/h. Most hypopneas might be classified as central because hypopneas were not associated with snoring, thoracoabdominal paradox, and increased inspiratory flattening of the nasal pressure flows signal during events [4]. Hypoxemia was mild (average oxygen saturation, 92.6%; desaturation to the nadir of 87%). The arousal index was elevated to 35/h, and three-quarters of arousals occurred with respiratory events. Abnormal leg movements were absent.
Neuropathological Findings
The autopsy revealed an almost complete neuronal loss in the substantia nigra, reactive gliosis, and microvacuolation. There was also some neuronal loss in the globus pallidus, subthalamic nucleus, thalami and brainstem nuclei. Moderate neuronal loss was presented in the locus coeruleus and inferior olivary nucleus, and mild loss was presented in the pontine tegmentum. Anti-phospho-TDP-43 antibodies reacted to surviving neurons.
DISCUSSION
PSG revealed irregular sleep-wake cycles, although the patient showed a nocturnal sleeping tendency. We can summarize the significant findings from PSG as follows; 1) highly fragmented and altered sleep architectures: disturbed non-REM (increased N1 and absent N3) but relatively preserved proportion of REM sleep, 2) abnormal breathing: hypoventilation and CSR while waking, and semi-periodic breathings similar to CSR, and obstructive sleep apnea mainly composed of central hypopnea while sleeping. Among the late symptoms of Perry syndrome, hypoventilation is the most dangerous because it may lead to respiratory failure and death [1]. Most patients with Perry syndrome have rapid, shallow breathing that alternates with normal breathing, hypopnea, or apnea [2].
The neuropathological findings are consistent with earlier reports of TDP-43 proteinopathy [5]. Complete neuronal loss in the substantia nigra was observed. The brainstem, especially the midbrain and pons, was the site of preferential neuronal loss.
Dopaminergic modulation of sleep-wake behaviors has been postulated: Dopamine promotes sleep through the basal ganglia networks but also promotes wakefulness through extra-basal ganglia circuits [6]. Besides respiratory disturbances, dopaminergic dysfunction through wide-spread neuronal loss in the substantia nigra, globus pallidus, subthalamic nucleus, and thalamus may contribute to the disrupted sleep-wake cycles of the patient. Meanwhile, the pedunculopontine nucleus, where cholinergic neurons reside in the pontine tegmentum, is involved in REM generation and maintenance [7]. Mild neuronal loss in that area might be associated with relatively preserved REM sleep.
The respiratory center is located in the medulla and pons. In this case, moderate neuronal loss presented in the locus coeruleus of the pons and inferior olivary nucleus of the medulla. In the medulla, the ventral groups of respiratory neurons (VRG) generate respiration, thus the bilateral VRG lesions caused a loss of automated breathing [8]. The dorsal raphe nucleus located on the midline of the midbrain and pons, which is sensitive to hypercapnia, projects to the VRG via serotonin [2]. An autopsy report had demonstrated the neuronal loss in the ventrolateral medulla and dorsal raphe nucleus in Perry syndrome [9]. CSR caused by brain stem structural lesions is also well known [10]. The impaired respiratory center located in the medulla and pons can be the neuropathological substrate of hypoventilation and CSR in Perry syndrome.
The patient had a tracheostomy. Six months later, he had a sudden unexpected death, which is frequently seen in Perry syndrome [1]. As a single-patient trial, a patient showing mild extrapyramidal symptoms but severe respiratory failure with Perry syndrome was inserted with a diaphragmatic pacemaker, and patient’s respiratory insufficiency was controlled [11]. However, the proper management of respiratory symptoms of Perry syndrome is unknown.