Sleep and Women’s Health
Article information
Abstract
Sex differences in sleep begin at a very early age and women report poorer sleep quality and have higher risk for insomnia than do men. Sleep may be affected by variation in reproductive hormones, stress, depression, aging, life/role transitions, and other factors. The menstrual cycle is associated with changes in circadian rhythms and sleep architecture. Menstruating women (even without significant menstrual-related complaints) often report poorer sleep quality and greater sleep disturbance during the premenstrual week compared to other times of her menstrual cycle. In addition to these sleep disturbances, women with severe premenstrual syndrome often report more disturbing dreams, sleepiness, fatigue, decreased alertness and concentration during the premenstrual phase. Sleep disturbances are also commonly reported during pregnancy and increase in frequency and duration as the pregnancy progresses. The precipitous decline in hormones and unpredictable sleep patterns of the newborn contribute to and/or exacerbate poor sleep and daytime sleepiness during the early postpartum period. Insomnia is also among the most common health complaints that are reported by perimenopausal women. Women are particularly vulnerable to developing insomnia disorder during these times of reproductive hormonal change. In this review, we present a discussion on the most relevant and recent publications on sleep across the woman’s lifespan, including changes in sleep related to menstruation, pregnancy, postpartum, and the menopausal transition. Treatment for sleep disturbances and insomnia disorder and special considerations for treating women will also be discussed.
INTRODUCTION
Research has shown that women report more sleep difficulties1,2 and are at greater risk for a diagnosis of insomnia compared to men.3,4 In the National Sleep Foundation’s 2007 poll, 30% of pregnant women and 42% of postpartum women reported rarely getting a good night’s sleep, compared with 15% among all women. Additionally, 25% of perimenopausal women and 30% of postmenopausal women reported getting a good night’s sleep only a few nights per month or less.5,6 In general, there is a higher prevalence of insomnia, restless leg syndrome, and dissatisfaction with sleep in women. In contrast, objective measures of sleep, measured by actigraphy and polysomnography (PSG), have demonstrated shorter sleep onset latency, increased sleep efficiency and total sleep time in women compared to men.7–9 Yet, a meta-analysis of sex differences of sleep behaviors in older adults (aged 58+) revealed no sex differences in total sleep time.10 Although sleep disturbances and insomnia disorder are widespread in the general population, each tends to occur more frequently in women, particularly during times of hormonal fluctuation. In addition to sex differences found in complaint of sleep disturbances and prevalence of sleep disorders, sex differences may also exist when treating men versus women. For example, in 2013 the U.S. Food and Drug Administration (FDA) required the manufacturers of Ambien to lower the recommended dose of zolpidem for women from 10 mg to 5 mg for immediate-release products and from 12.5 mg to 6.25 mg for extended-release products due to the risk of next-morning impairment and motor vehicle accidents. Women appear to be more susceptible to this risk because they eliminate zolpidem from their bodies more slowly than men. Zolpidem is the first drug in the U.S. to have different recommended doses for women versus men, but it seems likely pharmacokinetic sex differences would lead to differences in rates of absorption, metabolism, and excretion of other medications as well. Other biopsychosocial factors, such as discomfort during pregnancy, breastfeeding and infant/child care during the postpartum period, and potential ongoing nocturnal vasomotor symptoms (hot flashes and night sweats) during peri- and postmenopause, may complicate insomnia treatment and require special treatment considerations for sleep disturbances in women.
THE MENSTRUAL CYCLE AND MENSTRUAL CYCLE DISORDERS
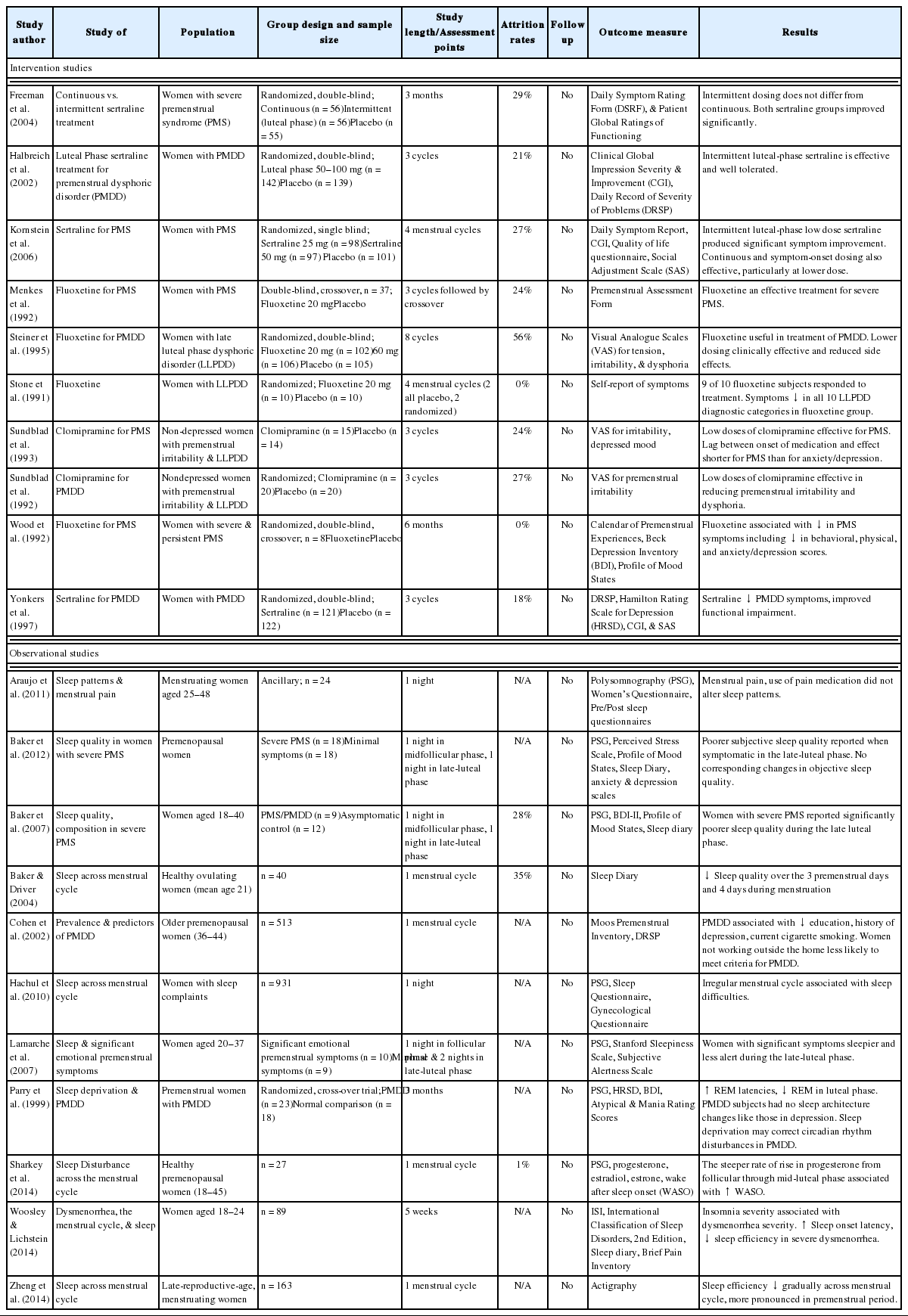
The menstrual cycle of healthy women is characterized by cyclic changes in production of estradiol, progesterone, lutenizing hormone, follicle stimulating hormone, prolactin, and growth hormone. Reproductive hormones not only regulate reproductive function during the menstrual cycle, but also influence sleep and circadian rhythms. Negative menstrual symptoms are most commonly experienced by women during the last few days of the cycle, as progesterone and estrogen levels decline.11 Premenstrual Syndrome (PMS) and Premenstrual Dysphoric Disorder (PMDD) are characterized by emotional, behavioral, and physical symptoms that occur in the premenstrual phase of the menstrual cycle, with resolution at the onset of menses or shortly thereafter. Many women of reproductive age experience some premenstrual symptoms, but 3–8% of women have clinically relevant premenstrual symptoms that they perceive as distressing and that affect daily function and meet diagnostic criteria.6,12,13 Women with PMS/PMDD typically report sleep-related complaints such as insomnia, frequent awakenings, non-restorative sleep, unpleasant dreams or nightmares, and poor sleep quality associated with their symptoms; and daytime disturbances such as sleepiness, fatigue, decreased alertness, and an inability to concentrate during the during the premenstrual week and during the first few days of menstruation.14–19 Women who experience severe premenstrual syndrome report significant declines in sleep quality in association with their symptoms during the late luteal phase compared with early follicular phase of their cycle.20,21 These corresponding changes, however, were not found in PSG sleep.22–24 Recently, actigraphic sleep was examined in participants from the Study of Women’s Health Across the Nations (SWAN) and investigators found that among later reproductive-age women, sleep efficiency declines across the menstrual cycle with the most pronounced decline in the last week of the menstrual cycle.25 Another recent study demonstrated that a steeper rate of rise in progesterone levels from follicular phase through midluteal phase was associated with greater PSG wake after sleep onset and sleep fragmentation in the late luteal phase.26 Sleep studies across the menstrual cycle have been limited by small sample sizes, heterogeneous cycle lengths, lack of ovulation timing controls, and oral contraceptive use. Due to these methodological issues and the limited nature of these studies, much remains unknown about premenstrual sleep.
Most women with PMDD seeking psychiatric help for this disorder present with symptoms of premenstrual depression, anxiety, and/or irritability. A number of treatment strategies currently exist that target these symptoms and appear beneficial in treating them.27 The selective serotonin reuptake inhibitors (SSRIs) fluoxetine and sertraline have been approved by the U.S. FDA for the treatment of PMDD. Fluoxetine,28–31 sertraline,32 and clomipramine33,34 appear to be highly effective for treatment of depression, however little data is available on the safety and efficacy of using SSRIs to treat sleep disturbance and insomnia in PMS and PMDD. Nonpharmacological interventions for insomnia, such as Cognitive Behavioral Therapy for Insomnia (CBTI), have not been empirically examined for premenstural insomnia. CBTI is a brief, structured, skill-focused psychotherapy aimed at changing maladaptive cognitions (i.e., thoughts and beliefs) and behaviors contributing to insomnia. The weight of evidence supporting CBTI, summarized in several meta-analyses,35–37 led to its recognition as a first-line treatment for insomnia by the NIH Consensus Statement.38 Improvements following CBTI are equivalent to those achieved during acute treatment with hypnotic medications39,40 and its effects are more durable after treatment discontinuation.39 Although efficacy has been demonstrated for adults with insomnia, it remains unclear if it is efficacious for women with PMS/PMDD and if special treatment considerations should be made (e.g., targeting other PMS symptoms such as menstrual pain41 or using CBTI skills intermittently during late luteal phase of a women’s menstrual cycle, as it is done to treat mood symptoms,42–44 when symptoms are likely to be the most problematic) (Table 1).
PREGNANCY
Pregnancy brings about significant fluctuations in hormones that affect the sleep-wake cycle and cause physiologic changes that lead to sleep disturbance. In addition to the hormonal changes, pregnancy itself causes a multitude of anatomic and physiologic changes; which are essential to maintain the pregnancy, but can also contribute to sleep problems. Common physical symptoms, such as anxiety, urinary frequency, backache, fetal movement, general abdominal discomfort, breast tenderness, leg cramps, heart burn, and reflux cause sleep disturbance during pregnancy. Complaints of sleep disturbance during pregnancy generally start at the onset of pregnancy and increase in frequency and duration as the pregnancy progresses due to pregnancy-related anatomic, physiologic, and hormonal changes.45,46 During the first trimester women tend to sleep longer and experience greater daytime sleepiness. Cross sectional and longitudinal studies that use subjective (self-report) and objective (PSG) measures of sleep have consistently documented increased wake after sleep onset and decreased sleep quality during the first trimester relative to pre-pregnancy.47,48 During the second trimester, daytime sleepiness improves. During the third trimester there is an increase in sleep disruptions with typically 3–5 awakenings per night, more daily naps,49 diminished daytime alertness, more disturbed dreams,50 and approximately 21% report disturbed sleep at levels consistent with a diagnosis of insomnia disorder.47,51 Decreased sleep efficiency, increased wake after sleep onset, increased total sleep time (decreased by third trimester), increased stage 1 and 2 sleep, and decreased rapid eye movement (REM) sleep (during late pregnancy) have been noted by PSG recordings.52–55 Poor and insufficient sleep during pregnancy are also associated with increased circulating levels of inflammatory markers involved in poor health56–60 and adverse pregnancy outcomes, including intrauterine growth restriction and preterm delivery.61–67 During the third trimester of pregnancy, insufficient and poor sleep may place women at increased risk for prolonged labor and cesarean deliveries68–70 and for having an infant small for gestational age (Table 2).71
For most women, sleep disruptions are caused by factors related to pregnancy, such as frequent need for urination during pregnancy.51 Some women, however, have difficulties initiating sleep and/or returning to sleep, which may be unrelated to peri-natal factors. When sleep disturbances are substantial (occur for 3+ nights per week for a period of 3+ months) and are associated with clinically significant distress or impairment of performance or other aspects of functioning, a diagnosis of insomnia disorder diagnosis is warranted. The prevalence of sleep disturbance among perinatal women is as high as 58%,72–74 and a probable diagnosis of perinatal insomnia is estimated at 10%.75 Daytime coping strategies such as napping, spending more time in bed, or increasing caffeine intake can perpetuate sleep difficulties. The presence of insomnia has a significant impact on quality of life and daytime functioning and its management is imperative.
Pharmacologic treatments, including sedative-hypnotics, benzodiazepines, and ramelteon for insomnia during pregnancy are typically avoided because of the potential for adverse effects such as low birth weight, preterm deliveries, and cesarean sections in pregnancy.76–78 Over-the-counter antihistimines and herbal and nutritional substances may be associated with fewer risks, but there have been fewer studies of their safety in pregnant women and their use is not recommended.79
Concerns regarding use of sleep medication during pregnancy and lactation make non-pharmacological treatment options for insomnia particularly attractive. Nonpharmalogical treatments such as CBTI should be the initial therapy. Current randomized clinical trials are under way to examine the efficacy and special considerations of CBTI during pregnancy. Additionally, studies of other nonpharmacological treatments options such as yoga,80,81 acupuncture,82,83 yoga combined with mindfulness,84 and exercise85 have been shown to be safe and effective treatments.
POSTPARTUM
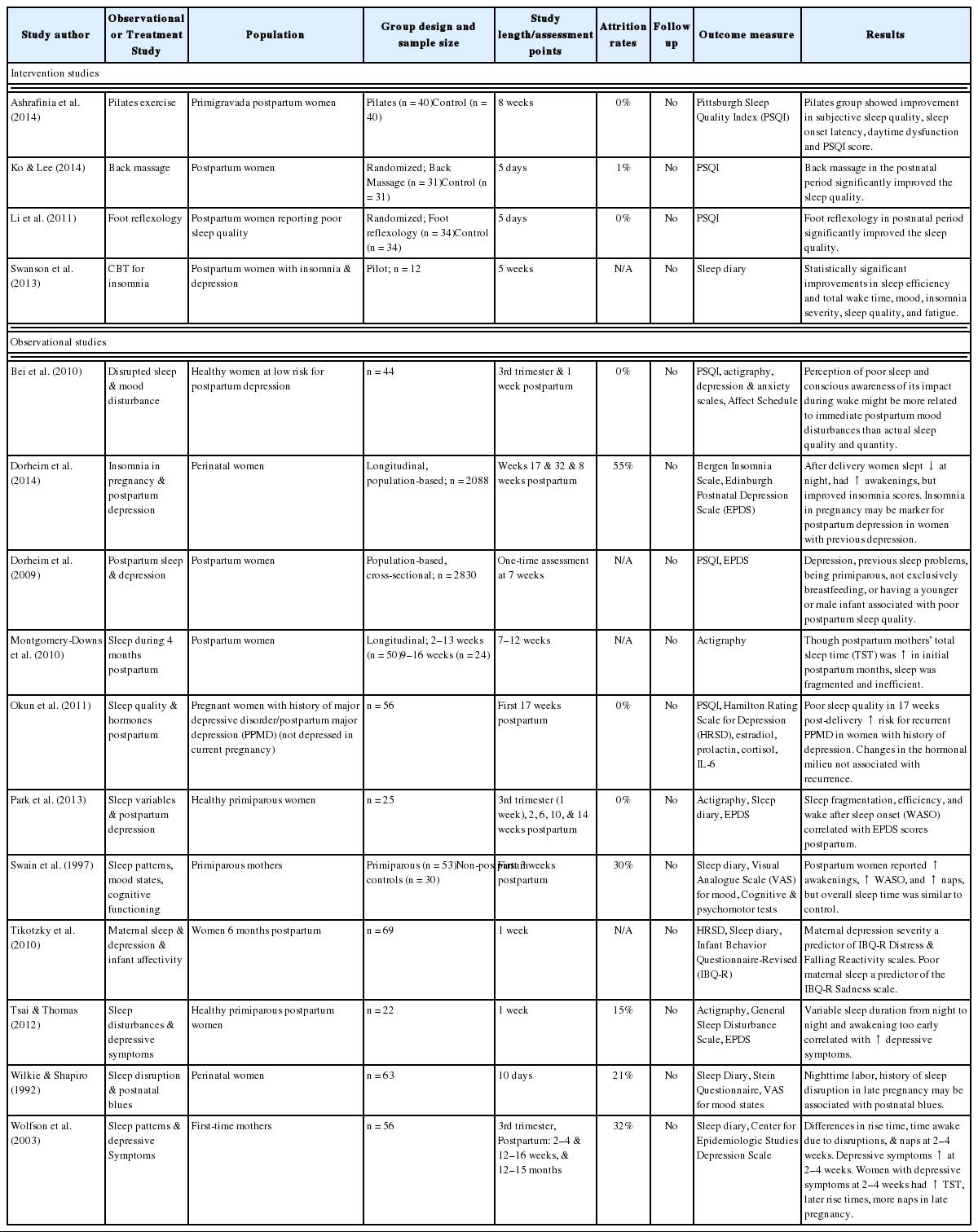
Sleep disturbance during the postpartum period and its effects on maternal role functioning and mother-infant interactions are not well understood (Table 3). Both self-report and actigraphy studies have demonstrated that nearly 30% of mothers have disturbed sleep after the birth of their baby. The precipitous drop in hormone levels after the birth and unpredictable infant sleep patterns can affect a new mother’s sleep. Longitudinal studies have documented that the first six months postpartum are associated with a significant increase in wake after sleep onset and a decrease in sleep efficiency compared to the last trimester of pregnancy.46,55,74,86,87 Fatigue and lack of energy remain high from pregnancy into postpartum period through the first year after delivery. Sleep begins to normalize around 3–6 months postpartum, around the time when infants begin distinguishing between day and night and sleep for longer periods of time during the night. Other factors such as the mother’s age, type of delivery, type of infant feeding, infant temperament, return-to-work issues, prior birth experience, number of other children at home, and availability of nighttime support from the partner or other family member can have an impact on quality and quantity of sleep in new mothers. Many women compensate for their sleep disruptions by spending more time napping during the early postpartum period.88
Negative effects of poor and insufficient sleep have been observed during the postpartum period. Mothers with poorer sleep (lower self-reported sleep quality and a higher number of night waking resulting from infant awakenings) perceived their infants as having lower mood and as being more distressed and tearful.89 Moreover, insufficient sleep and more time tending to the infant at night predicted poorer maternal-infant attachment. Several studies have documented the relationship between sleep disturbance and subsequent reports of depressive symptoms at a later time among perinatal women (later in pregnancy90–92 or in the early postpartum).91,93–96 The association between poor sleep and subsequent depressive symptoms also holds when sleep disturbance is experienced during the early postpartum period and postpartum depression develops at a later postpartum time.97–99
Interventions to improve maternal sleep and fatigue are limited, perhaps because of the universal nature of the experience and the belief that disturbed sleep is an unavoidable part of motherhood. In general, pharmacological interventions are seldom used in postpartum women who are breastfeeding. Even for women who are not breastfeeding, many choose not to take sedatives or other pharmacological options due to the need to have a more flexible sleep schedule for infant care. Therefore, behavioral interventions are the primary treatment options. Two pilot studies provide preliminary evidence for the efficacy of CBTI for postpartum insomnia and both studies demonstrated that the benefits of CBTI extended beyond improvement in sleep to other domains. One study provided five CBTI sessions, between the second and seventh postpartum weeks, to women who stopped smoking during pregnancy and found a significant decrease in time awake in the middle of the night and a significant increase in nocturnal (as well as per 24-hour) sleep time. Importantly, compared to women who did not receive the sleep intervention, those who did undergo CBTI had lower average daily cigarettes smoked and higher percent cigarette-free days.100 The second study provided CBTI to women with postpartum depression who also had disturbed sleep and reported pre to post treatment improvement in insomnia severity, sleep quality, sleep efficiency (% time asleep relative to time in bed), mood, and daytime fatigue.101 Studies of other nonpharmacological treatments such as reflexology,102 massage,103 and exercise104 have shown these options to be safe alternative treatments for postpartum women.
MENOPAUSE
Menopause is a natural process that occurs in women’s lives as part of normal aging. Menopause is defined as the cessation of menstruation due to degeneration of ovaries and follicles accompanied by changing ovarian hormone levels (estrogen and progesterone). The World Health Organization105 characterizes menopause as the permanent cessation of menstrual periods that occurs naturally or is induced by surgery, chemotherapy, or radiation. More recently menopause has been categorized in stages such as menopausal transition (defined by standardized criteria106 as variable cycle length seven days different from the normal cycle or > 2 skipped cycles and an interval of amenorrhea of 2–12 months) or postmenopausal (defined as > 12 months since last menstrual period). Menopause occurs between 50 and 52 years of age for Western women, but the range can vary based on race and ethnicity as well as lifestyle factors.107 The worldwide population of 470 million postmenopausal women is expected to increase, as 1.5 million women enter menopause each year, reaching a total of 1.2 billion by the year 2030.105 Most women now live long enough to become menopausal and can expect to live at least another 30 years beyond their final menstrual period (Table 4).
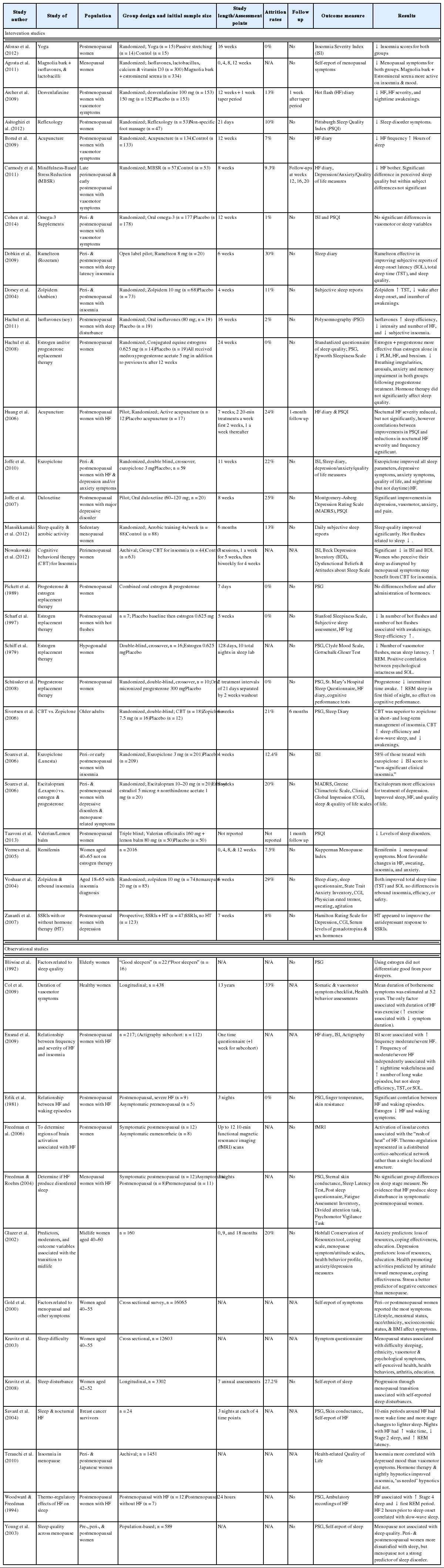
Many women go through the menopausal transition with few or no symptoms, while a small percentage of women suffer from symptoms severe enough to interfere with their ability to function effectively at home, work, or school. Common complaints include hot flashes, night sweats, insomnia, mood changes, fatigue, and excessive daytime sleepiness. In the 2005 NIH State-of-the-Science Conference panel report on menopause-related symptoms, sleep disturbance was identified as a core symptom of menopause.108 The prevalence of insomnia, defined as disturbed sleep associated with distress or impairment, is estimated at 38–60% in peri- and postmenopausal women.109,110 Troubled sleep was reported by 54–58% of women between 40 and 60 years of age in the Ohio Midlife Women’s study.111 The Wisconsin Sleep Cohort found that perimenopausal women and postmenopausal women were twice as likely to be dissatisfied with their sleep as premenopausal women.112 SWAN has shown that difficulty sleeping is reported by 38% of women between 40 and 55 years of age, with higher levels among late perimenopausal (45.4%) and surgical post-menopausal (47.6%) women.110
We are unable to find an estimate of prevalence on nocturnal hot flashes/night sweats; however, it is generally believed that hot flashes occur in 60% to 80% of women during the menopausal transition113 and persist for 4 to 5 years on average.114,115 When hot flashes occur during the night, they frequently awaken women from sleep; although not every nocturnal flash is associated with an awakening. Women with nocturnal flashes may also experience awakenings that are unrelated to a vasomotor event. Indeed, insomnia can occur during menopause independent of nocturnal flashes. Although self-reported nocturnal flashes correlate with poor subjective sleep quality, such association is less clear when objective sleep measures are used.112,116,117 There is only limited and contradictory evidence supporting an association between nocturnal flashes and sleep disturbance when both variables were measured objectively.112,116–122
The most common pharmacological treatments for menopausal insomnia include hormone replacement therapy (HRT), hypnotics and sedatives, and antidepressants. HRT is the primary treatment for menopausal symptoms, particularly vasomotor symptoms. The efficacy of HRT for sleep and mood disturbances remains unclear, with some studies finding positive results123–128 and others finding no benefit.129–131 Hypnotics and sedatives such as zalepon (Sonata), zolpidem (Ambien),132 and eszopiclone (Lunesta)127,133 have been shown to be effective in short-term use for acute, initial insomnia treatment in menopausal women. However, tolerance, withdrawal, dependence, and rebound depression at discontinuation may occur when drugs are used for longer than two weeks.134 Another psycho-pharmacological option is ramelteon, a selective melatonin receptor agonist, which has shown some efficacy.135 Antidepressant use has been effective in treating sleep disturbance in those with comorbid depression.136–139 Using antidepressants to treat sleep disruption in those without major depression, however, is not recommended.140 Herbal and dietary supplements such as black cohosh,141 omega-3,142 valerian,143 and isoflavens144,145 have gained popularity for the treatment of menopausal symptoms; however few studies have examined their direct effect on insomnia symptoms.
Hormonal fluctuation and vasomotor symptoms such as night sweats may be the initial cause of insomnia symptoms, but physiological arousals, behavioral conditioning, and misguided coping attempts appear to prolong insomnia.146 CBTI targets these behaviors and has been shown to be efficacious for the treatment of chronic insomnia in randomized trials of adults40 and in older adults.147 It may be beneficial for insomnia syndrome in menopausal women, however, to date, no randomized clinical trials have been conducted to examine efficacy of CBTI in menopausal women or special treatment considerations in this population. Preliminary data148 do demonstrate promising results for using CBTI for sleep disruptions affected by menopause. Other nonpharmacological options such as acupuncture,149,150 mindfulness,151 reflexology,152 exercise,153 and yoga154 have also shown some promise, however more evidence is needed to confirm their therapeutic benefits.
CONCLUSION
Sleep disturbances and disorders are common across a woman’s lifespan. Important biological events, often mediated by hormones and physiological changes, such as menstruation, pregnancy, and menopause commonly impact and often cause dissatisfaction with sleep. Given the fact that the negative impacts of poor sleep extend beyond tiredness and fatigue but also impair daytime functioning and mood, identification and treatment of these disorders is vital to a woman’s quality of life. Women looking to treat their sleep problems have many options from pharmacological help from drugs such as sedatives and hypnotics to HRT for those with menopause-related insomnia. Behavioral treatments such as CBTI offer longer-lasting improvements in sleep without the side-effects that are often accompanied by medications.
Despite advancing research in sleep and women’s health, there are several areas that deserve more focused research. Recently, there has been an increased interest on the menstrual cycle’s impact on the sleep cycle. While it is known that the hormones are linked to sleep and that the variability across the menstrual cycle causes changes in sleep quality, there are few studies that have explored treatment options in women with PMS and PMDD and significant sleep concerns. Additionally, though CBTI has been shown to be efficacious in treating chronic insomnia within various populations, including adult men and women and older adults, and among comorbid conditions like chronic pain and major depression, there is still a paucity of literature examining the efficacy of CBTI among women suffering from insomnia during times of reproductive change and special treatment considerations that may need to be taken into account. Future studies should include full-scale randomized trials of CBTI for women experiencing during the perinatal and perimenopausal periods. Treatment of sleep disturbances in women may have direct effects on quality of life as well as effects on mental and physical health.
Acknowledgements
We would like to thank Sooyeon Suh, PhD for her support and funding from National Institute of Nursing Research (grant #NR014008).
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.