Prevalence of Obstructive Sleep Apnea in 6–9-Year-Old Children of Visnagar: A Cross-Sectional Study
Article information
Abstract
Background and Objective
The prevalence of sleep-disordered breathing is extremely high, yet it is largely ignored. Significant adverse medical and psychological outcomes are associated with sleep-related respiratory disorders, including obstructive sleep apnea (OSA). Treatment and an early diagnosis will aid in averting additional severe consequences. Our study aimed to assess prevalence of OSA among 6–9-year-old children in Visnagar.
Methods
The approval of the school authorities and ethics committee was received. A sample size of 455 was determined. Clinical inspection was used to determine the prevalence of OSA in children aged 6 to 9 years using the “FAIREST-6” and BEARS criteria. The cases were classified as mild, moderate, or severe. By employing the chi-square test and Fisher’s exact test, inferential statistics were calculated.
Results
Prevalence of 8.13% (n = 37) among sample of 453 participants was observed. Gender predilection was found to be in 43.2% (n = 16) among females and 56.8% (n = 21) among males. Those diagnosed with OSA were categorized as mild 17 (45.94%), moderate 12 (32.43%), and severe 8 (21.62%).
Conclusions
OSA prevalence was determined 8.13% in the Visnagar population of children and adolescents aged 6 to 9 years using the FAIREST and BEARS questionnaire.
INTRODUCTION
Sleep plays a crucial role in the cognitive development of children. Its direct impact on contentment also extends to various aspects, notably growth, as evidenced by extensive research, particularly in the early stages of infancy [1]. The sleep requirements of individuals are strongly correlated with their age. Newborns (0 to 3 months) are recommended to have 14 to 17 hours of sleep per day, while infants (4 to 11 months) should aim for 12 to 15 hours. Toddlers (12 to 35 months) need 11 to 14 hours, preschoolers (3 to 5 years) should have 10 to 13 hours, and school-aged children (6 to 13 years) require 9 to 11 hours of sleep [2].
Insufficient sleep during early childhood has been linked to a range of health issues, according to the American Academy of Pediatrics (AAP). These include allergic rhinitis, compromised immune function, anxiety, depression, as well as cardiovascular complications such as obesity, diabetes, and hypertension [3].
In the pediatric population, sleep disorders can be classified into two categories: insomnia and parasomnia. Parasomnia is characterized by rhythmic movement disorders, head banging or rocking, sleep onset difficulties, inadequate sleep syndrome, snoring, and obstructive sleep apnea (OSA) [4].
Adenotonsillar hypertrophy, along with craniofacial dysmorphism, obesity, and hypotonia, is acknowledged as a significant etiological element in OSA [5]. It is correlated with a decline in overall well-being and manifests in physical manifestations such as fatigue, parched mouth, and headaches, as well as mental complications including depression [6,7].
OSA is commonly misdiagnosed during initial examinations. Consequently, essential questionnaires such as the Epworth Sleepiness Scale (ESS) questionnaire [8], the Berlin Questionnaires [9], Pittsburgh Sleep Quality Index [10], and others can be employed to establish an initial diagnosis of OSA. Mild or moderate OSA can be categorized based on these scales. Similar to well-established “gold standards” for diagnosing pediatric sleep disorders, Functional Airway Evaluation Screening Tools (FAIREST) [11] and BEARS [12] can be utilized.
Constricted airways that exacerbate sleep apnea and malocclusion, in addition to the development of attention deficit hyperactivity disorder and developmental delays, and heavy mouth breathing resulting in retrognathia or an elongated facial structure, are among the long-term complications of sleep apnea that may persist into adulthood [6]. Depending on the severity and underlying conditions that contribute to upper airway obstruction during sleep, various treatment modalities are frequently combined, including weight loss, orthodontic and medical interventions, myofunctional therapy, and adenotonsillectomy [13].
Numerous population-based studies have been conducted and published on the prevalence of sleep-disordered breathing (SDB) and its effects. Within the present article, we have provided a comprehensive summary of the epidemiology of SDB in children across different populations [14]. Anuntaseree et al. [15] explored the prevalence of OSA in children aged 6–13 years in Hat Yei, Southern Thailand, which was found to be 0.69%. This figure closely resembles the rates observed in Western populations. Schlaud et al. [16] estimated the prevalence of OSA to be 3.2% among 9–10 year old children attending primary schools in Hannover, Germany. Furthermore, Goyal et al. [17] estimated that the prevalence of OSA in school-aged children aged 5 to 10 years from three randomly selected schools in Bhopal, India was 9.6%.
There have been limited investigations conducted in the Indian subcontinent to examine the occurrence and associated complexities of OSA among children. Consequently, we devised this study in order to ascertain the prevalence of OSA within the Visnagar Taluka of Gujarat, India.
METHODS
This prospective cross-sectional observational study was conducted in Visnagar, Gujarat, between March and June 2022. The Ethics Committee of the College of Dentistry Research Centre at University approved the study (NO: NPDCH/IEC/2022/373/1). Informed consent from parents was taken.
Calculation of Sample Size
Based on a 95% confidence level and a statistical power calculation of 85%, the necessary sample size of 473 was estimated, assuming a 19% prevalence of SDB in Visnagar and 0.25 nonresponses.
Selection of Schools
The Ministry of Education provided a comprehensive inventory of all public and private primary schools in Visnagar that educate boys and girls. The primary schools hold the responsibility of imparting knowledge and instruction to pupils ranging in age from 6 to 12 years old. Schools for this survey were selected using stratified randomization to ensure that the sample was representative of Visnagar’s school population.
A number was assigned to each public and private school in Visnagar (North, East, West, South, and Central). Following this, schools were chosen at random from a randomization table. Ten schools in total were chosen, 5 of which were public and 5 privates; one public and one private school per district catered to both males and females.
Requesting the principals of the schools to partake in this research was accomplished through a letter that detailed the study’s objectives and requested their consent for the distribution of the questionnaires. In order to select minors, 480 questionnaires were made available in Visnagar.
A sampling procedure analogous to that employed for school selection was utilized to determine which classes and pupils would attend each of the selected schools. The investigation excluded children who were diagnosed with on going orthodontic treatment, underwent adenoidectomy, neuromuscular disease, congenital heart disease, or coronary disease. The FAIREST (https://www.fairest.org/tools/) and BEARS questionnaire (https://www.medicalhomeportal.org/clinical-practice/screening-and-prevention/screening-for-sleep-problems) were utilized in the OSA evaluation process. They incorporated a cover page that described the significance of investigating the subject and the purpose of the study. There was an emphasis on the confidentiality of all information pertaining to the individual. The survey gathered pertinent demographic and personal data, including the parents’ educational attainment, school name, district, and gender. This segment of the survey also evaluated the existence of orofacial symptoms and comprised inquiries regarding the habits of sucking the digits, gnashing the teeth both during the day and at night, discomfort in the temporomandibular joint, and pain in the facial muscles.
Categorization
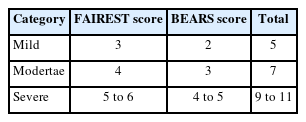
Categorization was done based on combination of FAIREST and BEARS scores (Table 1).
Statistical Analysis
The data was systematically gathered and organized in an Excel spreadsheet prior to conducting a statistical analysis utilizing SPSS version 20.0 (IBM Corp., Armonk, NY, USA). The descriptive data was presented as a percentage. By employing the chi-square test and Fisher’s exact test, inferential statistics were calculated (p-value = 0.05). Chi-square test was used to determine the age-wise categories of OSA. Fisher’s exact test was performed to determine the gender predilection.
RESULTS
A total of 455 children underwent intraoral examinations, comprising 227 males and 228 females. Responses to the questionnaire for all participants were received (100% response rate). It was observed that 8.13% of participants were diagnosed with OSA (Table 2). Among which majority were of age between 7 to 8 years, predisposing more in males (56.75%) when compared to females (Table 3).
Table 3 shows frequency of severity of OSA in children. Among which 17 (45.94%) had mild, 12 (32.43%) had moderate, and 8 (21.62%) had severe OSA.
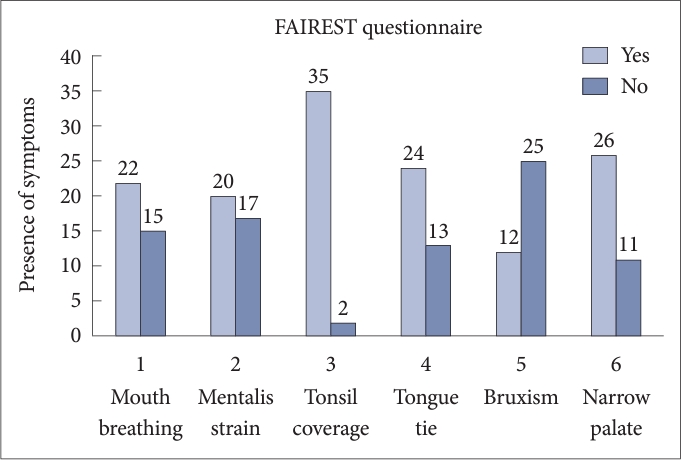
In this study, among all domain of FAIREST scale, symptoms which strongly associated with high risk of SDB were tonsil coverage (94.59%) and narrow palate (70.27%). Other common symptoms were observed such as mouth breathing (59.45%), mentalis strain (54.05%) and tongue tie (64.86%). Least commonly seen symptom was bruxism (32.43%) (Fig. 1).
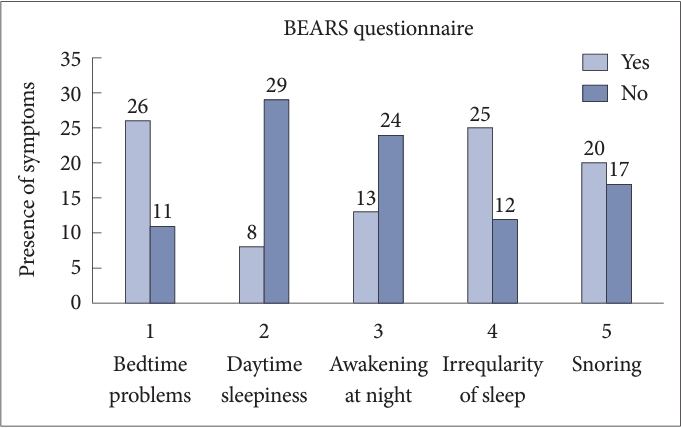
Among BEARS questionnaire, the most commonly observed symptoms were bedtime problems (70.27%), irregular sleep patterns (67.56%), and snoring (54.05%). Least frequency of symptoms associated with OSA were daytime sleepiness (21.62%) and nighttime awakening (35.13%) (Fig. 2).
DISCUSSION
Concern is increasing regarding the prevalence of OSA in children due to its potential impact on oral health and development. Early intervention in the treatment of sleep difficulties in children has the potential to positively influence their academic performance, overall well-being, and health. A comprehensive sleep screening may assist in the identification of academic, behavioral, health, and quality of life concerns [18].
There have been various investigations conducted in an attempt to diagnose OSA. In terms of prognosis, polysomnography (PSG) is superior. However, it should be noted that PSG is exceedingly costly and necessitates a minimum of two consecutive nights of data collection. Furthermore, for an accurate analysis of sleep patterns, it is necessary to sleep in a specialized clinic or hospital, and under certain circumstances, the waiting list may extend for up to a year.
A sleep technician is required to be present throughout the entire duration of this type of evaluation. One of the primary concerns regarding PSG is its perceived unsuitability for continuous sleep monitoring. Portable sleep monitoring (PSM) devices present themselves as compelling alternative diagnostic techniques due to their exceptional diagnostic accuracy in symptomatic moderate/severe OSA. The availability of well-validated PSM devices and their utilization in the hands of dedicated and qualified sleep physicians may decrease the sole reliance on laboratory-based PSGs for the effective management of clinically severe OSA cases. However, inadequate case selection combined with the increased utilization of these devices by untrained professionals would worsen the issue of delaying or misdiagnosing simple OSA and the treatment of other sleep-related breathing disorders such as complex sleep apnea, upper airway resistance syndrome, sleep-related hypoventilation, and so forth [19]. Approximately 90% of general practitioners do not employ OSA screening techniques, and nearly half of them neglect to screen individuals with an increased susceptibility to developing the disorder. Consequently, employing a study based on questionnaires may prove to be the most appropriate approach for the preliminary and initial diagnosis of OSA in children [20].
In previous studies that employed the BEARS questionnaire to identify obstructive sleep apnea (OSA) in children, however, it was observed that while the BEARS questionnaire was effective in detecting sleep disturbances, it did not provide any information about other clinical factors that are associated with OSA [21-23]. On the other hand, in studies that utilized the FAIRST questionnaire to diagnose OSA in children, the authors reached the conclusion that it is a concise, validated tool that solely relies on clinical evaluation [11,24].
Therefore, in order to determine sleep disruptions and clinical variables connected to the occurrence of OSA, the current study implemented the utilization of the BEARS and FAIRST questionnaires. Consequently, it is postulated that these surveys serve as a more dependable and accurate tool for evaluating the susceptibility of OSA [11,21].
Considerable research is disseminated in order to ascertain the prevalence across diverse ethnic and racial populations. Pediatric OSA is extremely prevalent, impacting 1%–4% of the general pediatric population, per the American Association of Pediatric Dentistry in 2002. The prevalence of OSA in the Chinese population was determined to be 4% [25]. Shin et al. [26] conducted a cross-sectional survey of 15–18-year-old high school pupils in the southern region of Seoul, Korea. They deduced that the prevalence of OSA was 11.2% overall. Ng et al. [27] conducted a questionnaire-based study among children aged 6 to 12 years in Hong Kong and discovered that the prevalence of OSA was 10.2%.
The investigation conducted by Anuntaseree et al. [15] aimed to determine the prevalence of OSA among adolescents aged 6 to 13 years in Hat Yei, Southern Thailand. Their findings revealed a prevalence rate of 0.69%.
Pattanaik et al. [12] utilized the STOP-Bang questionnaire to establish the prevalence of OSA, which was found to be 13.7%. Through the utilization of sleep-related breathing disorder questionnaires, Goyal et al. [17] discovered a prevalence rate of 9.6% for OSA in infants. In 2008, Suri et al. [28] conducted a questionnaire-based survey to assess the prevalence of OSA among school-aged children residing in the National Capital Region (NCR), encompassing the Delhi metropolitan area as well as Faridabad, Gurgaon, Ghaziabad, and Noida. The survey determined an overall prevalence rate of 4.8% for OSA. In line with previous research, the current study, utilizing FAIREST-6 and BEARS questionnaire scores, determined the prevalence of OSA among children aged 6 to 9 years to be 8.13%.
Bruni et al. [29] published a Sleep Disturbance Scale for Children (SDSC) that was used to assess sleep irregularities in children aged 5.8 to 15.3 years. However, the SDSC’s main emphasis was questions concerning sleep disturbances and behavior, with only three questions about SDB (snoring, breathing difficulties throughout the night, and inability to breathe during sleep) raised. Furthermore, it showed limitation of these questionnaires which had not been tested against a rigorous overnight PSG research. Owens et al. [30] created the Children’s Sleep Habits Questionnaire (CSHQ), a parent-reported sleep-screening instrument for school-aged children. However, this questionnaire had the flaw of only including a small number of items about sleep-related respiratory issues. Chervin et al. [31] validated a Pediatric Sleep Questionnaire on 162 children aged 2–18 years for sleep-disordered breathing, snoring, drowsiness, and behavioral disorders.
To ascertain the prevalence of objective and subjective factors associated with OSA patients, numerous studies were conducted. In their study of children aged 5 to 12 years, Blader et al. [32] employed the BEARS screening instrument and found that 27% of the participants reported having bedtime problems. In contrast, Owens and Dalzell [13] reported that only 16% of the children experienced bedtime problems. The current study observed a threefold increase in bedtime difficulties, amounting to 70.27%. In other studies that also employed the BEARS screening instrument, Mohammadi et al. [21] identified a prevalence of snoring in 10.53% of children diagnosed with OSA, while Baidas et al. [22] reported 14.4%.
The present investigation reveals a significantly elevated incidence of snore, precisely 54.05%. In children, Kang et al. [33] discovered a correlation between adenotonsillar hypertrophy and OSA. Similarly, our research demonstrates a considerable incidence of tonsillar hypertrophy, accounting for 94.59% of cases. In addition, our findings indicate that a high narrow arch palate is a factor in the development of OSA, with a prevalence of 70%; this is consistent with the result (71.11%) reported by Victor [34].
The studies have identified various symptoms that are similar, such as adenotonsillar hypertrophy, tongue tie, high narrow arch palate, snoring, and respiratory difficulties during sleep. These symptoms have been found to be contributing factors and are associated with OSA.
In order to determine the applicability of our findings to all children from North Gujarat, it is necessary to conduct additional research. The study’s comprehensiveness would be enhanced by including participants from diverse cities within the region.
Limitation
The findings of the current study, as obtained through the implementation of a questionnaire, are subject to the subjective interpretations of parents, thereby presenting a limitation. It is crucial to acknowledge a range of contextual factors when evaluating this study, including but not limited to the age demographic, the size of the sample, the participants’ ethnicity, the variables being analyzed, and the design of the questionnaire.
Conclusion
For the purpose of screening, prevalence of OSA was determined through the use of the FAIREST and BEARS questionnaires, which was 8.13% among 6–9-year-old children, with no gender predilection found. Furthermore, an evaluation has been conducted on the exceedingly common symptoms, which comprise adenotonsillar hypertrophy, tongue tie, snoring, and high narrow arch palate. To ensure an accurate diagnosis, additional inquiry is necessary.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: Shoba Fernandes, Yash Bafna. Data curation: Zeel Shah, Swetal Agrawal. Investigation: Zeel Shah, Swetal Agrawal. Methodology: Zeel Shah, Swetal Agrawal. Project administration: Zeel Shah, Swetal Agrawal. Supervision: Shoba Fernandes, Yash Bafna, Dharati Patel, Dimpal Parmar. Validation: Shoba Fernandes, Yash Bafna, Dharati Patel, Dimpal Parmar. Writing—original draft: Zeel Shah, Swetal Agrawal. Writing—review & editing: Zeel Shah, Swetal Agrawal.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Funding Statement
None
Acknowledgements
None