INTRODUCTION
There are two basic categories of sleep: non-rapid eye movement (NREM) and rapid eye movement (REM). NREM is composed of three stages, with each stage representing progressively deeper sleep. Slow wave sleep (SWS) is defined as the sum of stage 3 and stage 4 NREM sleep and is characterized by highvoltage, synchronized electroencephalographic (EEG) waveforms, often measured as slow wave activity [1]. SWS plays an important role in cerebral restoration and recovery in humans and is involved in the maintenance and consolidation of sleep [2]. SWS is also essential for homeostasis and feeling revitalized upon waking. It serves as a restorative factor in the central nervous system [3] and facilitates the clearance of metabolites [4]. During SWS, slow fluctuating neural oscillations/brain waves are followed by combined waves of blood and cerebrospinal fluid, impacting neural activity [5]. Indeed, sleep and particularly SWS is important for cognitive function [6]. Additionally, SWS can increase the level of arousal-promoting neuromodulators and decrease accumulated metabolites [7].
However, the exact nature and roles of SWS are not clearly understood and there is still much to learn about SWS generation and its physiologic functions. Tononi and Cirelli [8] examined the electrophysiologic character of SWS and demonstrated its important role in synaptic strength, leading to improved memory and cognitive processing [9,10]. Still, information regarding the prevalence of SWS in different populations, especially patient populations, is incomplete and scarce [11,12].
SWS deficits have been observed in patients with schizophrenia [13], major depression disorders [14], fibromyalgia [15], posttraumatic stress disorder [16] and people with generalized anxiety disorder [17]. The reduction of SWS has also been observed among older people [18] and those with insomnia [19]. However, few studies have examined the prevalence of SWS in people with obstructive sleep apnea (OSA), who often complain about non-deep sleep and exhaustion. Though Afifi et al. [20] found abnormalities in cortical arousal activity in people with OSA, they did not assess SWS. Catcheside and Jordan [21] found that the respiratory arousals (RAs) from a state of sleep to wakefulness during SWS were strongly correlated with the oxygen desaturation index. Additionally, Ren et al. [22] showed that patients with OSA experienced less SWS. However, it was not clear whether the reduction in SWS was related to abnormal breathing or the extent of OSA as determined by the minimum oxygen saturation.
In general, the two broad areas of abnormalities studied in OSA include cognitive function and psychomotor performance [23], while the relationship between oxygen saturation and SWS has received less attention. Previous research indicated that compared to healthy people, those with OSA exhibited some abnormality in brain activity during NREM and REM sleep. Moreover, brain activity was positively correlated with apnea-hypopnea index (AHI) and oxygen desaturation index [24]. In a metaanalysis, Mullins et al. [25] claimed that while findings were inconsistent, overall, OSA patients had fewer and slower sleep spindle frequency activities compared with the controls.
To our knowledge, there is no study that has investigated the relationship between SWS and minimum oxygen saturation in OSA despite documented associations between brain activity and AHI [26]. Therefore, the present study aimed to examine the relationship between SWS and minimum oxygen saturation in patients with OSA based on the polysomnography (PSG) scores. It was hypothesized that 1) there will be a significant difference in SWS scores across mild, moderate, and severe apnea cohorts, 2) there will be a positive correlation between SWS and oxygen saturation, and 3) oxygen saturation will predict SWS.
METHODS
Participants
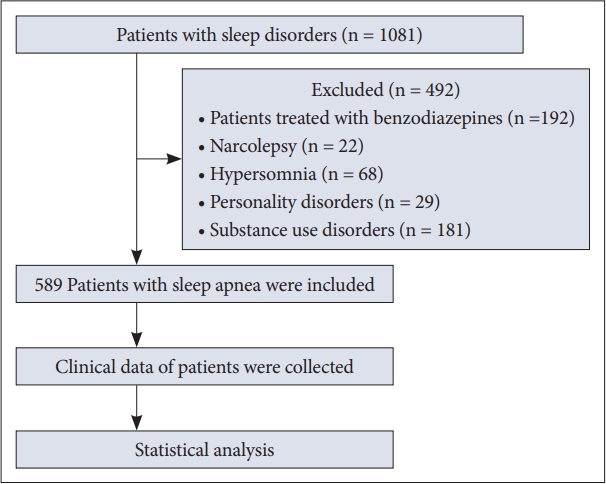
The participants were 589 Iranian adults with OSA who were patients at the Sleep Disorders Research Center (SDRC), Farabi Hospital, Kermanshah University of Medical Sciences (Kermanshah, Iran). They underwent PSG to evaluate abnormalities of sleep and/or wakefulness and other physiologic disorders. The inclusion criteria were: 1) AHI score ≥ 5, 2) ages < 65 years, and 3) adults living in Iran. Exclusion criteria were: 1) personality disorders, 2) substance use disorders, 3) patients treated with benzodiazepines, GABA-receptor agonists, or opiates, 4) narcolepsy, 5) hypersomnia, 6) chronic fatigue syndrome, and 7) severe bipolarity with psychotic symptoms, suicidal ideation and intent, or severe sensorimotor injury (Fig. 1). The exclusion criteria were designed to mitigate the possibility of recruiting participants with depression, insomnia, and other related conditions that could have confounded the study analysis.
Procedure
Full-night PSG recordings of 589 patients with sleep-disordered breathing (SDB) who had been studied between April 2019 and June 2022 were retrospectively analyzed. The sample comprised 210 females and 379 males; with a mean age of 48.54 years (SD = 13.61). Patient data included weight, height, body mass index, respiratory disturbance index (RDI), AHI, arousal index, total sleep time, and periodic leg movements. Like the AHI, RDI reports on respiratory distress events during sleep, but unlike the AHI, it also includes respiratory-effort related arousals.
A full-night PSG was performed in the SDRC at Kermanshah University of Medical Sciences (Kermanshah, Iran). Patients’ charts and permission to use this data for research purposes were provided by the local Institutional Review Board, provided that patient confidentiality would be strictly respected (Ethical Approval ID: IR.KUMS.REC.1401.360). The research presented in this manuscript was conducted according to the principles of the Declaration of Helsinki [27].
All individuals had undergone overnight PSG with recordings of EEG, electrooculogram, bilateral pretibial electromyogram, and electrocardiogram. Nasal airflow was measured via impact pressure through a nasal sensor in which pressure fluctuations of the breathed air stream were determined.
Thoracic and abdominal excursions, oxyhemoglobin saturation (pulse oximeter), and body position were simultaneously recorded. The polysomnographic recordings were performed using the Alice-LE-Diagnostic Sleep System (SOMNOmedics GmbH, Randersacker, Germany). For each patient, PSG was performed in a standard way between 10 pm and 6 am. In the morning, following the night of study, sleep stages and sleep-related respiratory events were manually scored according to the American Academy of Sleep Medicine 2012 guidelines [28].
A nasal airflow amplitude reduction greater than 90%, lasting for at least 10 s, was defined as apnea. Hypopnea was defined as an airflow reduction between 50% and 90% with an associated 3% reduction of blood oxygen saturation (SpO2). Further classification in obstructive, central, or mixed respiratory apnea events was based on simultaneous evaluation of nasal airflow and thoracic and abdominal excursion.
The AHI is an index used to indicate the severity of sleep apnea. It represents the number of apnea and hypopnea events per hour of sleep. An apnea is a pause in breathing that lasts at least 10 s. Hypopnea is a period of shallow breathing or decreased airflow. The AHI is calculated by dividing the number of apnea events plus hypopnea events by the total number of minutes of sleep, then multiplying this number by 60 to get an hourly rate. The severity of sleep apnea is classified based on AHI as follows: normal sleep, AHI < 5; mild sleep apnea, AHI ≥ 5 but < 15; moderate sleep apnea, AHI ≥ 15 but < 30; severe sleep apnea, AHI ≥ 30. Consequently, in this study, there were three analysis AHI groups (AHI score 5–15 for mild apnea group, 16–30 for moderate apnea group, and > 30 for severe apnea group). Using the PSG device software, the RA number during each individual’s sleep period as well as the duration and amplitude of each RA were visually registered; like the AHI, it reports on respiratory distress events during sleep, but unlike the AHI, it also includes respiratory-effort related arousals. RAs were detected in 30-s epochs and were scored using the American Academy of Sleep Medicine criteria. Intraclass correlations for both SWS duration and AHI intensity were calculated.
Statistical Analysis
We compared PSG data and demographic information between the three groups via a series of independent one-way analysis of variance (ANOVA) tests. Moreover, the relationships between SWS and minimum oxygen saturation were examined via a series of Pearson’s correlations and a multiple linear regression test. All statistical analyses were conducted with SPSS version 25 for Windows (IBM Corp., Armonk, NY, USA). Statistical significance (p value) was set at alpha < 0.05.
RESULTS
Table 1 provides an overview of the demographic background of the participants for the three identified AHI cohorts: mild, moderate, and severe apnea (AHI score 5–15 for mild, 16–30 for moderate, and > 30 for severe). Additionally, the one-way ANOVA results are provided, indicating significant differences across groups for virtually all variables, except height, total time of sleep, wake index, and periodic leg movements, and arousal.
To test the first hypothesis, the mean score for SWS, which is a percentage of the total sleep time was examined across the three sleep apnea cohorts. As highlighted in Table 2, ANOVA results suggested there was a significant difference across the three groups (p = 0.05). The highest SWS mean score (15.70) was observed in the moderate apnea group. The second-highest SWS score (13.59) was observed in the mild apnea group, and the lowest SWS score (11.75) was observed in the severe apnea cohort. However, post-hoc calculations (Bonferroni tests) showed a significant difference only between the moderate and severe groups (mean difference = 3.95, p = 0.04).
The second hypothesis, that there would be a positive association between SWS and oxygen saturation, was tested using a Pearson’s correlation coefficient, and revealed a significant relationship (r = 0.23, p < 0.001). Finally, for the third hypothesis, that oxygen saturation would predict SWS, a multiple regression (Table 3) was performed using the predictor variables of minimum oxygen saturation.
The regression and ANOVA outputs indicated that the regression models were statistically significant and suggested that minimum oxygen saturation (standardized coefficients = 0.23, t = 5.11, p = 0.001) significantly predicted variance in SWS.
DISCUSSION
This study aimed to evaluate the relationship between SWS and blood oxygen saturation in 589 patients with OSA based on the PSG scores. The study findings supported the hypotheses.
In this study, it was hypothesized that there would be a significant difference in SWS scores among groups of mild, moderate, and severe apnea. Results indicated that SWS was significantly lower in the severe AHI cohort than in the moderate AHI group. These data are consistent with previous findings about SWS in patients with apnea [21,22,29].
SWS plays an important role in memory and cognitive function, and reduction in fatigue [9]. In other words, SWS plays an important role in homeostasis, cerebral restoration, and recovery [30,31]. In patients with severe apnea, the amount of SWS is reduced, as shown in our results (Table 2). Therefore, it is reasonable to propose that increasing oxygen saturation may be beneficial to those with apnea. There are several ways to increase blood oxygen saturation such as continuous positive airway pressure [32]. There also appears to be a strong relationship between SWS and reduced arousal from sleep. Arousal from sleep is dependent on sleep–respiration interactions in different stages of sleep. Notably, autonomic activation and higher cortical arousal have been reported in patients with apnea [33]. SWS helps to reduce arousal events [34]. Clinical data have shown that respiratory events, as well as RAs, are most prevalent in lighter stages of NREM sleep [35]. Arousal microarchitecture could be a key to understanding respiratory events. Furthermore, there is evidence that duration and intensity of RAs correlate with PSG-related disease severity in patients with apnea [36]. Therefore, it may be helpful in clinical practice and research to consider SWS as a target for methods to counteract arousal events in patients with apnea.
Regarding the second hypothesis, we expected a positive association between SWS and minimum oxygen saturation scores, and the data supported this assumption. Moreover, the findings were aligned with those of the previous studies that demonstrated a similar connection [21,22,37]. In the present study, results indicated that as the oxygen saturation decreases, the amount of SWS decreases. For instance, the lower minimum oxygen saturation (mean score = 72.80%) was observed in the severe apnea cohort. Indeed, prior research has found that when people with sleep apnea enter SWS, the amount of apneic events increases due to the decrease in oxygen desaturation during sleep [38]. Therefore, this could be a reason for the reduction in SWS in people with apnea.
Based on recent imaging studies, in people with apnea, some brain regions such as the anterior cingulate, frontal cortical, and brainstem areas are subject to structural changes [39]. Previous studies suggested that the arousal stimulus in NREM is related to the level of inspiratory effort. Therefore, mechanisms of arousal in sleep apnea might also play a role in the relationship between SWS and oxygen saturation [40,41].
Finally, our third hypothesis was supported by the findings. The present results showed that lower oxygen saturation is associated with lower SWS. It appears that different factors that increase the breathing effort, (e.g., hypercapnia and hypoxia), as well as mechanical respiratory events during sleep, trigger an RA which could explain this connection [8,42]. In particular, oxygen desaturation seems to play an important role in levels of SWS. A previous study [43] indicated that the maximum oxygen desaturations (SaO2) during respiratory events with arousals are larger than the desaturations in events without arousals. Moreover, the sleep stages seem to play an important role in oxygen desaturation.
Limitations
The present study had several limitations. First, confounding variables such as depression, insomnia, and medical conditions may have influenced the study outcomes. While inclusion and exclusion criteria were used to mitigate the potential for these confounds, such conditions often co-occur and might have impacted the results. Second, in the present study, we did not provide specific details regarding the duration of reduced oxygen saturation. This information is important for a comprehensive understanding of the effects being investigated. Future research should include detailed information on the duration of reduced oxygen saturation, to provide a more complete picture. Lastly, AHI is a widely used metric, but it primarily focuses on the number of apneas and hypopneas per hour of sleep and may not fully capture the complex nature of sleep apnea. Therefore, it is important to consider other measures and clinical assessments to provide a more comprehensive evaluation of sleep apnea severity.
Conclusion
There was a significant positive correlation between SWS and oxygen saturation in people with sleep apnea. Lower SWS scores were associated with lower oxygen saturation. Furthermore, oxygen saturation significantly predicted SWS. Given the role that SWS plays in cognitive function and reduction in fatigue and arousal events, the clinical ramifications of these findings are worthy of consideration. Our findings imply that oxygen saturation interventions could helf enhancing SWS duration.