INTRODUCTION
Obesity is an important health care issue. It is one of the most important risk factors for some chronic diseases such as hyperlipidemia, hypertension, type-2 diabetes, and certain types of cancer which may cause disabilities and increase mortality rates. However, its trend is increasing especially among women living in developing countries [1,2]. Obese individuals usually have a lot of co-morbidities including sleep disturbances and depression. The prevalence of sleep disturbances is high among obese individuals. Rose et al. [3] reported that about 25% of obese people suffer from sleep disturbances. Liu et al. [4] indicated that obesity is a moderator in association between sleep disturbances and metabolic syndrome including diabetes. Similarly it has been shown that obesity plays a moderator role in association between sleep disturbances and depression.
Depression is a prevalent disorder so that up to 2030, it will be the second most important source of burden of disease and disabilities after ischemic heart disease [5]. The current prevalence of depression in national studies was reported to be about 4.1%, while lifetime depression prevalence is even much more. Furthermore, women are 1.95 times more probable to have depression compared to men [6,7]. The prevalence of circadian rhythm dysfunction is high among patients with depression [8].
On the other hand, obesity mostly correlated with depression [9,10]. The level of this correlation depends on gender and varies in different levels of body weight. The depression is mostly observed among women and it is deteriorating with increasing of body mass index (BMI) [11-13]. The odds ratio of depression is increased approximately by two folds in obese females compared to those with healthy weight [12,14]. The risk of developing the symptoms of depression such as persistent feeling of sadness, loss of interest, change in sleep pattern, and appetite is increased approximately 37% in women with BMI > 30 [13].
Sleep disturbances are also common in individuals with depression and obesity [15]. Circadian rhythm disturbances may be responsible for this comorbidity [16,17]. Germain and Kupfer [18] explained the role of circadian rhythm in pathogenesis and treatment of depression. On the other hand, Engin [19] revealed that disturbance in circadian rhythm may change eating habits so that it may cause an increase in body weight. Unfortunately, coexistence of sleep disturbances with depression signs and obesity complicates the treatment of each and decreases the compliance and response to treatment [13]. Depression is usually an exclusion criteria in most previous clinical trials of obesity, and obesity co-morbidity is usually ignored in the clinical studies on treatment of depression. Also, even with known medications for decreasing depression, less than 50% (and even in some countries less than 10%) of patients with depression use these medications due to depression stigma, or unwillingness to intake psychiatric medications in some individuals [20]. Therefore, finding a safe dietary supplement that could simultaneously target obesity, sleep disturbance and depression is very important.
Recently, beyond the monoaminergic hypothesis of depression, melatonergic hypothesis has been raised [21]. Melatonin is the regulatory hormone for sleep-wake cycle that secretes from the pineal gland into the blood stream [22]. Another hypothesized role of melatonin is adjustment of metabolic homeostasis, dietary intake and body weight via an specific neuroendocrine system [23]. Researchers at cohort study of 15655 residents at West Washington observed a strong correlation between melatonin supplement intake and weight loss or weight control (avoiding weight regain after weight loss) [24]. Melatonin supplement has been administrated in some other psychiatric conditions and it has been reported to be safe [22,25].
The aim of this study was to evaluate the melatonin effects in women with obesity/overweight co-morbid with mild and moderate depression, and sleep disturbance seeking weight reduction in a placebo controlled randomized clinical trial (Clinical trial registration number: IRCT2014020316465N1).
METHODS
Participants
The clients in a private weight reduction clinic assessed for eligibility to take part in this 12-week parallel, double-blind, placebo-controlled randomized clinical trial. Inclusion criteria were: 18 to 50 years old women, BMI Ōēź 25, diagnosis of mild and moderate depression by Beck Depression Inventory (BDI) and semi structured clinical interview for Diagnostic and Statistical Manual of Mental Disorders-fifth edition (DSM-5) criteria for depression based on Schedule for Affective Disorders and Schizophrenia, diagnosis of insomnia based on DSM-5 criteria for sleep-wake disorders [26]. Exclusion criteria: bipolar and related disorders, severe depression or suicidal thoughts, pregnancy and lactation, menopause, hypothyroidism, diabetes, and taking antidepressant and sleep medications, appetite and metabolism altering medications. We also ruled out the primary insomnia like sleep apnea and insomnia due to other medical and environmental conditions or drug use by interview and we tried to include only insomnia that could be attributed to depression.
Sample size
The sample size was determined with SigmaPlot 12 sample size calculator (Systat Software Inc., San Jose, CA, USA) in type I error ╬▒ = 0.05 and with a power of 1-╬▓ = 0.80 to detect 5 kg mean difference between groups with standard deviation of 5.4 based on pilot study. Based on this, the sample size of 20 patients in each group was calculated to be sufficient.
Procedure
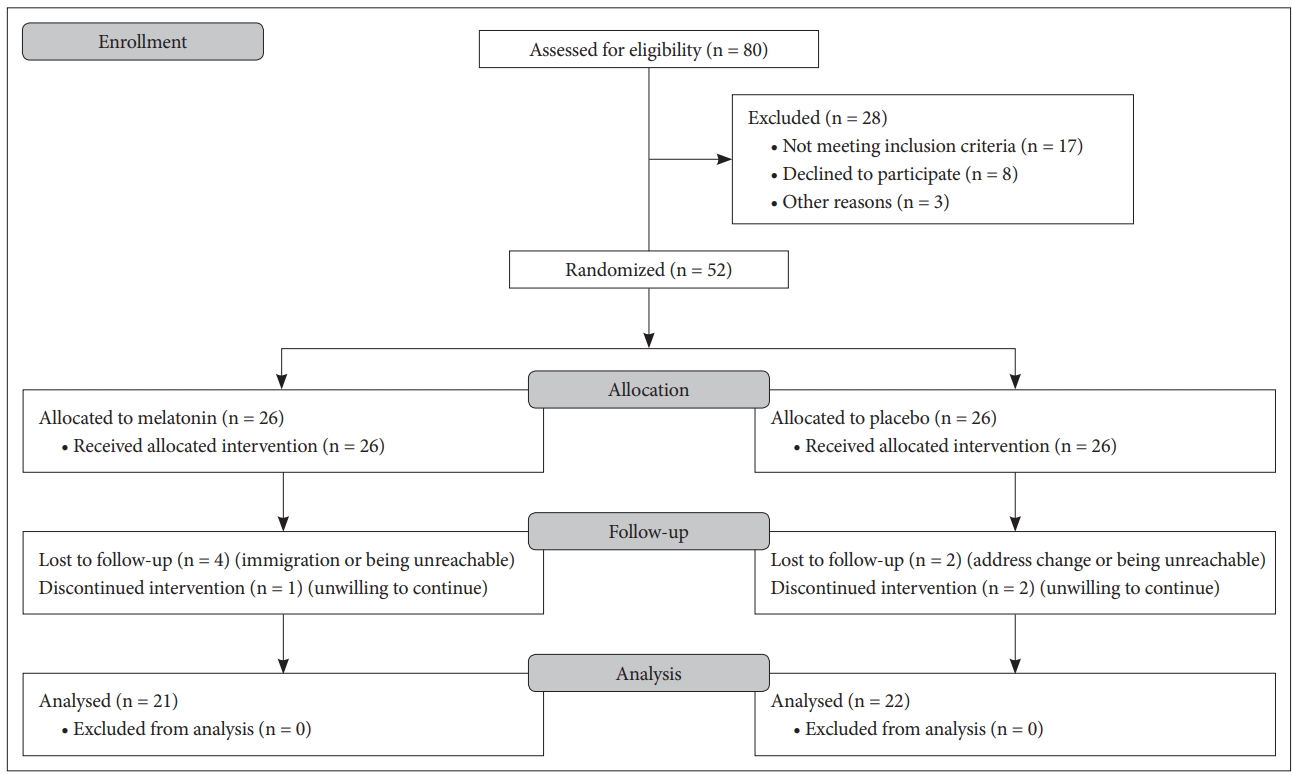
Eighty clients of a private weight reduction clinic were assessed for eligibility and study procedure was described to them. Fifty two patients with sleep disturbance, and mild or moderate depression who had the BMI greater than 25 signed the informed consent form and enrolled. Fig. 1 shows the CONSORT flow diagram of the study. The participants were randomly assigned into one of the two groups receiving 3 mg melatonin tablet (one hour before bedtime) or similar placebo made by the same factory (one hour before bedtime). In order to generate the random allocation sequence, we used the computerized simple random allocation method. Allocation to treatments was done by concealment of the groups to participants and researchers using placebo and encoding. The codes were kept by a third party and only decoded after the analysis finished. Random allocation sequence and subject enrollment were also performed by a third party. The diagnosis of depression and insomnia performed by a psychiatrist using semi structured clinical interview based on DSM-5 criteria. Furthermore, the BDI was used to discriminate the mild/moderate depression.
The participants in both groups were received a similar weight reduction diet with -500 to -1000 kcal of their usual dietary intake prescribed by a clinical dietitian. We instructed the participants to fill the 3-day food diary forms and deliver in each visit to check their compliance to their dietary plan and the study protocol. Also, all participants were advised to keep their usual physical activity and we asked it in every visit. Anthropometric measurements, clinical validated body composition assessments [27], BDI [28], Spielberger State-Trait Anxiety Inventory (STAI) [29], and Pittsburgh Sleep Quality Index (PSQI) [30] performed at the baseline visit, and repeated 2 weeks, 4 weeks, 8 weeks, and 12 weeks after the start of the study. One month after stopping the supplementation at the follow up visit, weight was measured again to compare weight regain between the two groups.
Study tools
Pittsburgh Sleep Quality Index
The PSQI is an instrument to assess sleep quality by measuring seven factors including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction. Each factor could score between 0 to 3 points which ŌĆ£0ŌĆØ indicates no difficulty and ŌĆ£3ŌĆØ indicates sever difficulty in sleep. The scores of all components are summed to produce a total score ranging from 0 to 21. The highest score indicates worst sleep quality and lower score indicates better sleep quality. The cutoff value of 5 could discriminate clients with insomnia from normal clients with sensitivity and specificity of 94% and 72%. The CronbachŌĆÖs alpha coefficient for the scale was 0.77 [31].
Beck Depression Inventory second edition
BDI is a 21-items questionnaire and is the most widely used self-report inventory to measure the symptoms and severity of depression in adults. Previous studies assessed the psychometric characteristics of this scale and reported CronbachŌĆÖs alpha of 0.87 and acceptable test-retest reliability (r = 0.74) [32].
Spielberger State-Trait Anxiety Inventory
This instrument contains 40 Likert-type questionnaires assessing state and trait anxiety in adults. The first 20 items assess state anxiety and the second twenty items assess trait anxiety. Abdoli et al. [33] assessed reliability and validity of this scale and reported an acceptable internal consistency with CronbachŌĆÖs alpha of 0.886 for trait anxiety and 0.846 for state anxiety.
Statistical analysis
At the end, data gathered and analyzed with analysis of variance (ANOVA) with repeated measures and null hypothesis of similarity of weight (as primary outcome), sleep quality score, and depression symptoms (as secondary outcome) was tested between groups and during the study period. We used the intention to treat analysis approach with last observation carried forward procedure. If a participant had lost to follow up before the third session of measurements (week 4) with any reasons she would have dropped out from the analysis, but if she had exited from the study after the week 4, its data would have remained in the analysis and missing data would have imputed using the last observed carried forward technique.
The statistical analyses were performed with SPSS 19 software (IBM Corp., Armonk, NY, USA) and figures were plotted with SigmaPlot 12 software.
Ethics
The ethical approval was received from ethic committee board of Tehran University of Medical Sciences before patientŌĆÖs enrollment (ethics code: 22516). Furthermore, patients were required to sign the informed consent forms before enrolment and they were free to leave the study at any level with any reason. This study was conducted in accordance to the last version of Helsinki statements in ethical standards for medical research involving human subjects.
RESULTS
Finally, 21 patients in the melatonin group and 22 patients in the placebo group completed at least 4 weeks according the study protocol and entered into the analysis (the dropout rate before the week 4 was 17.3% and after that nearly 5%).
Mean age and BMI of analyzed subjects was 37 years ┬▒ 9.7 and 33 ┬▒ 5.4, respectively. There were no significant differences between groups regarding demographic, anthropometric, and clinical variables at baseline (Table 1). Comparison of body weight, BDI score, sleep quality score, and STAI between the melatonin and placebo in each trial week is shown in Table 2.
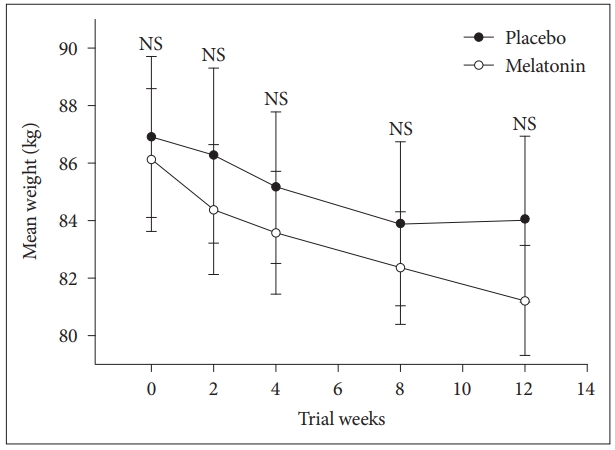
Mean difference ┬▒ standard deviation of body weight reduction in melatonin group was -4.8 kg ┬▒ 5.1 compared to -3.4 kg ┬▒ 3.3 in placebo group. The difference between groups was not significant using independent sample t-test (t(41) = -1; p = 0.280; 95% confidence interval [CI]: -4 to 1.2). We performed repeated measures ANOVA to compare the main effects of melatonin and placebo and the interaction effect between groups during the study on body weight in subjects with co-morbidity of obesity sleep disturbance, and mild and moderate depression. Subjects on the melatonin reduced more weight compared with the placebo but difference between groups was not significant, F = (1, 41) = 0.2, p = 0.650. The interaction effect of group and time was not also significant F = (1, 41) = 0.81, p = 0.410 (Fig. 2).
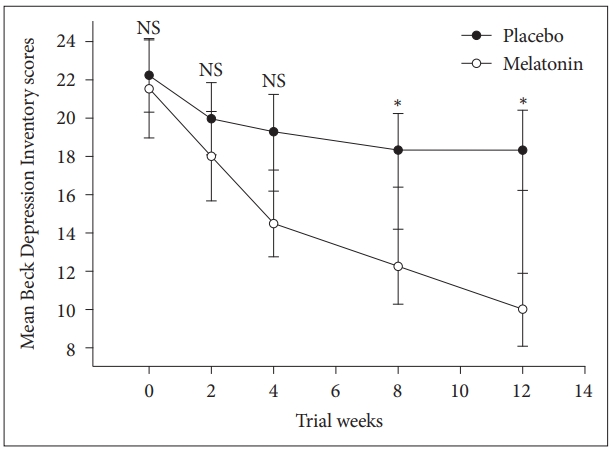
We performed repeated measures ANOVA to compare the main effects of melatonin and placebo and the interaction effect between groups during the study on depression score in subjects with co-morbidity of sleep disturbance, obesity and mild and moderate depression. Melatonin significantly reduced depression symptoms compared with the placebo, F = (1, 34) = 6.2, p = 0.017. The interaction effect of group and time was also significant F = (1, 34) = 6.6, p = 0.004 (Fig. 3).
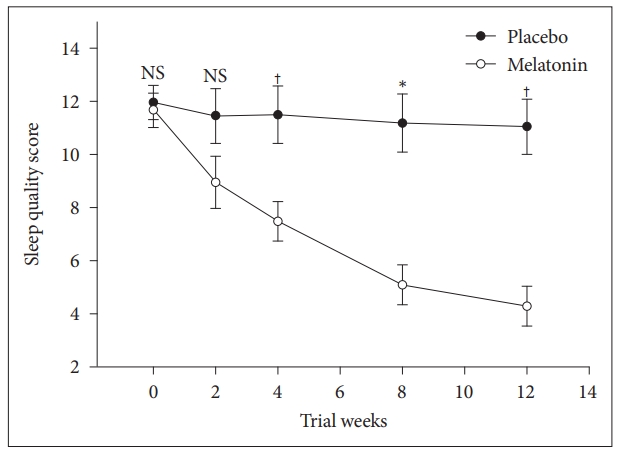
We also performed repeated measures ANOVA to compare the main effects of melatonin and placebo and the interaction effect between groups during the study on sleep quality score in subjects with co-morbidity of sleep disturbance, obesity and mild and moderate depression. Melatonin significantly reduced sleep quality score, F = (1, 33) = 8.0, p = 0.008. The interaction effect of group and time was also significant F = (1, 33) = 13.9, p < 0.001 (Fig. 4).
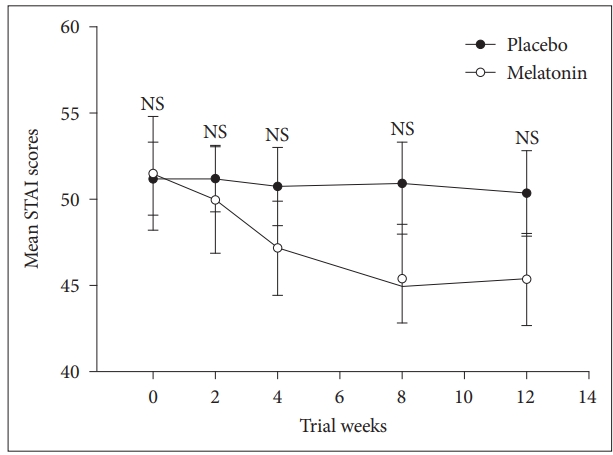
Furthermore, we performed repeated measures ANOVA to compare the main effects of melatonin and placebo and the interaction effect between groups during the study on STAI score in subjects with co-morbidity of obesity, sleep disturbance, and mild and moderate depression. Melatonin clinically reduced anxiety but different between groups was not significant, F = (1, 40) = 0.7, p = 0.390. However, the interaction effect of group and time was significant F = (1, 40) = 4.7, p = 0.012 (Fig. 5).
At the follow up visit, one month after finishing the melatonin supplementation, we observed 1.3 ┬▒ 1.9 kg weight regain, while in the placebo group we observed 0.29 ┬▒ 3.7 kg weight regain (t(23) = 0.79; p = 0.400).
Four cases reported minor side effects in the melatonin group (two cases reported the headache, one case reported the drowsiness during day time, and one case reported the nightmare). Also, four cases reported side effects in the placebo group (two cases reported the nausea, one case reported the headache and one case reported the insomnia). Also no withdrawal syndrome was observed.
DISCUSSION
The main finding of this study was that melatonin at the dose of 3 mg nightly reduced depression symptoms significantly compared with the placebo. Some investigators have reported a very strong relationship between circadian rhythm and depression. Melatonin and its derivative agomelatine acts as an antidepressant agent with its activity both as an agonist at MT (1) and MT (2) receptors and antagonist at 5-HT (2C) receptors [34]. Similar to our findings, Kasper et al. [35] in a clinical trial of agomelatine in patients with major depressive disorder studied the effects of agomelatine compared with sertraline. In this 6-week study, 154 patients with depression received 25 to 50 mg agomelatine and 159 patients received 50 to 100 mg sertraline. At the end of the study symptoms of depression, anxiety, and sleep quality significantly improved (p Ōēż 0.05) in the group receiving agomelatine. As well, we observed decrease in anxiety score after 12 week melatonin supplementation but this reduction was not statistically significant compared to the placebo. Hansen et al. [36] also performed a clinical trial on effects of 6 mg melatonin on depression, anxiety and sleep disturbances in patients undergoing breast cancer surgery. Melatonin could significantly reduce the risk of depressive symptoms compared to placebo during a 12-weeks period after breast cancer surgery. In contrast to our results, Dolberg et al. [37] administered slow released melatonin adjunct with fluoxetine in 10 patients with major depressive disorder and found no significant difference in severity of symptoms of depression compared with fluoxetine and placebo (n = 9). The antidepressant effects of fluoxetine may have overshadowed the effects of melatonin.
We also observed significant better sleep quality indicated by decreased PSQI during the consumption of melatonin. Xu et al. [38] studied the effects of 3 mg melatonin administration in patients with primary insomnia. In spite of improvement in some aspects of sleep, they reported no effect of melatonin on PSQI. One of the main reasons for this conflict could be difference in the type of insomnia in two studies. Xu et al. [38] included the patients with primary insomnia and excluded the cases with depression. However we included the clients with secondary insomnia due to depression. Shahrokhi et al. [39] treated the patients with cancer by 6 mg melatonin for 30 days and reported a significant improvement on PSQI scores. It seems that the effects of melatonin on sleep may vary by factors like primary or secondary insomnia, co-morbid diseases, melatonin dose, and duration of intake.
In regard with body weight, Rastmanesh et al. [40] in 2012 proposed that melatonin may have the potential to prevent and even treat obesity. In animal studies, melatonin effects on eating behavior and body weight were promising. Some evidences show that melatonin can influence on the regulation of energy metabolism and body weight in rats. Some other studies have confirmed that melatonin can inhibit fat accumulation in fat tissues of obese animal models. Montano et al. [41] administered 1 mg/kg bw for 4 weeks and investigated its effects on feeding behavior of non-diabetic male Wistar rats. They observed that administration of external melatonin may reduce food intake, appetite, abdominal fat and body weight. Prunet-Marcassus et al. [42] also observed that Melatonin can reduce body weight gain in rats with diet-induced obesity and also decrease obesity metabolic side effects. They proposed that melatonin is involved in the regulation of body weight. All of these studies were performed in animals and our study was the first human trial of melatonin which specifically was designed to assess the melatonin effects on body weight of patients with mild and moderate depression. We used the minimal dose of melatonin. Maybe more dosages of melatonin lead to different results.
To the best of our knowledge, up to now there was not a published human clinical trial of melatonin effects on body weight of patients with mild and moderate depression. However, there are a few human studies performed on melatonin effects on body weight in some other neurologic conditions [22,25]. A very recent meta-analysis on these studies have concluded and proposed that melatonin may have buffering or regulatory effect on body weight [43]. When the background disorder causes increase in body weight and insomnia melatonin could slow down this complication and where the background disorder causes weight loss melatonin was able to slightly attenuate this effect. This is consistent with our results. At the follow up visit after finishing the melatonin supplement, we observed 1.3 ┬▒ 1.9 kg weight regain that further put emphasis on the regulatory role of melatonin in body weight control.
In the present study, we hypothesized that if melatonin could help to lose body weight, this effect might have been more detectable in subjects with co-morbidity of obesity, insomnia and depression. So we designed our study and proposed that melatonin may help to lose body weight through improving sleep disturbances and reducing depression symptoms. In designing the study, we choose to include weight reduction seeking women with co-morbidities of obesity, sleep disturbance and mild and moderate depression. We tried to eliminate the effects of different diets by prescribing similar -500 to -1000 kcal of regular dietary intake in both groups and we tried to control for most known and unpredicted confounding factors by randomization, to be able to detect the pure effect of melatonin on body weight. The findings of our study show that melatonin does not help to reduce body weight significantly, despite significant improvement in sleep quality score, and depression symptoms compared to placebo.
Furthermore, melatonin did not impose any serious side effects in comparison with the placebo. Similarly, Hansen et al. [36] also at the dose of 6 mg melatonin in patients undergoing breast cancer surgery did not report any serious side effects compared with placebo.
Limitations
Generalizability of the findings is one of the limitations of this study. The findings of this relatively small and focused sample study should be interpreted with caution for larger implications for society and population sleep health. Furthermore, another limitation of this study is that objective sleep tests such as polysomnography, were not assessed in this study. To compensate for this limitation we ruled out the primary insomnia like sleep apnea and insomnia due to other medical and environmental conditions or drug use by interview and we tried to include only insomnia that could be attributed to depression.
Conclusion
Based on our findings melatonin (at the dose of 3 mg one hour before bedtime) is not effective in reducing body weight, but as a safe over the counter supplement, it is helpful in patients with co-morbidities of sleep disturbance, mild/moderate depression and overweight/obesity in improving sleep quality and reducing the symptoms of depression. Slight weight regain after finishing the melatonin supplementation, might point at the regulatory role of melatonin in body weight. We encourage that future investigations put this point at their spotlight. No serious side effects and no withdrawal syndrome were observed in administration of melatonin at 3 mg/nightly dose. Hence, obese people with co-morbidities of mild and moderate depression and poor sleep quality may benefit from low dose melatonin.