INTRODUCTION
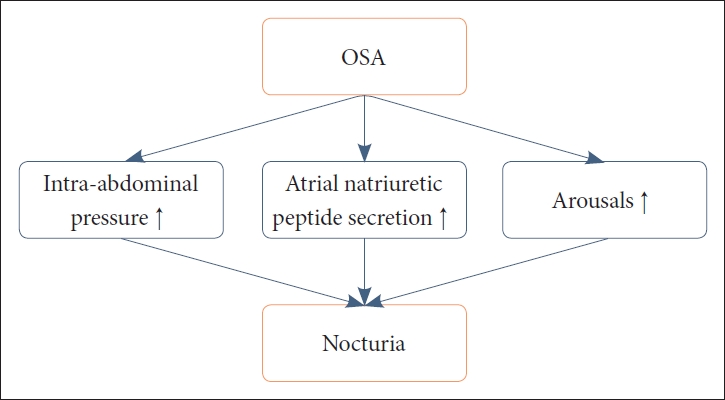
Nocturia is a lower urinary-tract symptom (LUTS) that is defined by the International Continence Society as the sensations to void one or more time during the night [1]. It is closely related to sleep problems [2], and the bidirectionality of the link between sleep and nocturia is often discussed [3]. The prevalence of nocturia has been reported to be 11% to 35.2% in men aged 20–40 years, but notably escalated prevalence rates are evident in the elderly population, with rates ranging from 68.9% to 93% [4]. In an internet-based survey on the general population aged ≥ 40 years, prevalence of nocturia was 76.1% for women and 74.0% for men [5]. Nocturia is a clinically notable culprit for sleep problems, with reports of a significantly proportional increase in the number of nocturnal voids and a close correlation with poorer sleep quality [6]. Moreover, detrimental effects on daytime functions and increased prevalence of excessive daytime sleepiness were noted in patients with nocturia [6,7]. Furthermore, it is usually a challenge for sleep specialists to evaluate whether nocturia precedes insomnia or vice versa. The exact mechanisms underlying the bidirectionality between the two clinical constructs remain elusive. Despite its clinical importance, history taking, proper evaluation, and management of nocturia are frequently dismissed by sleep clinicians. In explaining the association between sleep and nocturia, much of the recent evidence has centered on the relationship between obstructive sleep apnea (OSA) and sleep. Indeed, mounting evidence emphasizes nocturia as an important clinical indicator of OSA. Increments in intra-abdominal pressure, increased atrial natriuretic peptide secretions, and arousals due to OSA may be mechanisms that result in nocturia and resultant poor sleep quality (Fig. 1) [8]. We will mainly discuss common comorbid conditions of nocturia that clinicians face in clinical settings and their relationships to sleep and OSA.
COMMON COMORBID CONDITIONS OF NOCTURIA AND THEIR RELATIONSHIPS TO SLEEP AND OSA
OSA, Nocturia, and Sleep
The relationships between OSA, nocturia, and resultant sleep disturbance have been reported in many previous studies. The purported mechanism for the frequent nocturia observed in OSA patients is hypoxia induced by OSA and the resultant sequential events of negative pressure breathing and atrial natriuretic peptide secretion, which ultimately lead to nocturia episodes [9]. In a recent meta-analysis on the relationship between OSA and nocturia, there was a 1.5-fold increase in the risk of nocturia in men, but no significant relationship was noted in women [10]. According to the aforementioned meta-analysis, such a risk was not changed by the methods implemented to diagnose OSA [10]. Indeed, when assessed with the Berlin Questionnaire, there was an approximately 3-fold increase in the events classified as high risk of OSA in the nocturia group over those for the controls [11]. Another report with polysomnography (PSG) data reported a higher risk, with a 2.4-fold increase of incidence of severe OSA in patients with a nocturia history [12].
Moreover, interestingly, one study reported that nocturia might be as sensitive as snoring or body mass index (BMI) in the prediction of OSA [13]. One recent study suggested that OSA patients with nocturia represented a clinically severe OSA phenotype, with higher apnea-hypopnea index, more severe excessive daytime sleepiness, lower nadir oxygen levels, and higher BMI [12]. Another study reported a linear increase in the severity of OSA with the frequency of nocturia [14]. Another study recommended further urological evaluation in OSA patients with fewer compromised oxygen desaturation events even after correction for OSA [15]. Moreover, interestingly, not the severity or length of hypoxia but the intermittent desaturation events were predictive of nocturia incidence in sleep disordered breathing (SDB) patients in one study [16], probably because the respiratory efforts to compensate for limited airflow lead to increments in abdominal pressure that induce nocturia [16].
In the findings that attest to the fixed relationship between nocturia and OSA, the effectiveness of continuous positive airway pressure (CPAP) in relieving nocturia has been replicated. With one-year treatment of CPAP, most urologic parameters, including nocturia episodes, nocturnal polyuria prevalence, and detrusor overactivity all demonstrated significant improvements [17]. CPAP effectively improved daytime sleepiness, depression, and quality of life in OSA patients with nocturia [18,19]. Nocturia frequency decreased in terms of urine volume, which largely affected quality of life in OSA patients [20]. More significantly, nocturia was predictive of hypertension in OSA patients, and CPAP therapy was recommended as relieving both nocturia and hypertension in one study [21]. SDB patients with nocturia who were responsive to CPAP therapy all showed positive outcomes, with decreased systolic blood pressure B-type natriuretic peptide [22]. More studies are pointing to the importance of the relationship between coronary heart disease and nocturia [23]. Thus, nocturia in OSA patients should not be underdiagnosed in clinical settings, considering its importance in the prevention and management of cardiovascular diseases.
Benign Prostate Hyperplasia: Relationship to Nocturia, Sleep, and OSA
Patients with benign prostate hyperplasia (BPH) were prone to complain of poorer sleep quality, which was proportionally worsened by nocturia episodes [24]. One PSG result on 20 patients with BPH demonstrated that BPH patients experienced nocturia mostly during superficial sleep stages or rapid eye-movement sleep [25]. Nocturia in BPH patients was significantly associated with excessive daytime sleepiness [25], probably because sleep continuity can be disrupted by nocturia [26]. Severe insomnia and poor sleep quality were noted in BPH patients who experienced frequent nocturia episodes [24,27].
A single-therapy group of tamsulosin hydrochloride, an alpha-1-blocker, and a combination-therapy group of tamsulosin hydrochloride and meloxicam, a cyclooxygenase inhibitor-2, were compared to evaluate their effectiveness in improving sleep quality in BPH patients with nocturia [28]. The combination therapy significantly decreased episodes of nocturia and improved sleep quality [28]. Melatonin helped BPH patients with nocturia by reducing habitual mid-night awakenings [29]. Another study on patients with LUTS suggestive of BPH demonstrated that those who were refractory to alpha-1 antagonist therapy, when it was combined with ramelteon, a melatonin 1 and 2 agonist, had their nocturia episodes significantly reduced without adverse events [30]. Moreover, BPH patients with partial remission of nocturia when receiving tamsulosin (alpha-1 antagonist) were responsive to combination therapy with zolpidem. Zolpidem’s effect on nocturia and sleep was implemented not only by exerting its gamma-amnobutyric acid (GABA)-ergic effect on sleep induction but also by suppression of urine excretion [31]. BPH patients after surgical intervention, usually transurethral resection of the prostate, experienced alleviation of sleep disturbances and nocturia [32]. Patients with residual symptoms after transurethral resection tended to have voiding and storage problems [32].
In patients with BPH, frequency of nocturia was proportionally increased with the odds of having symptoms of OSA [33]. BPH with frequent nocturia episodes should be evaluated for OSA. Moreover, one study also reported that BPH patients with storage symptoms were highly likely to have OSA [34]. In a study that evaluated the relationship between CPAP adherence and nocturia episodes in BPH patients, there was no significant relationship between the two [35]. It was assumed that disconnection of the mask because of nocturia would affect CPAP adherence, but that pattern was not as evident in BPH patients who perceived therapeutic benefits from CPAP therapy [35].
Overactive Bladder: Relationship with Nocturia, Sleep, and OSA
Recent literature demonstrates the importance of understanding the relationship between overactive bladder (OAB), nocturia, and sleep, since nocturia is the most common LUTS of OAB [36]. According to one study on OAB symptoms in 150 women, the most frequent complaint was “strong desire to void,” with mean nocturia episodes of 4.5 [37]. All women with OAB and nocturia complained of insomnia [38], and another study found that sleep disturbances and fatigue were evident in most OAB patients, with such complaints linearly increasing with severity of OAB symptoms [39]. Decreased quality of life and significant sleep disturbances were observed especially in OAB patients who awaken more than twice during the night [40]. When compared with insomnia patients, OAB patients tended to wake up at night with shorter duration, because of a strong urge to void at night [41]. OAB symptoms frequently result in urgent urinary incontinence, which can lead to poor sleep quality and excessive daytime sleepiness [42].
An effective treatment can benefit OAB patients to a significant degree, with improvements in sleep parameters [43]. Mirabegron, a beta-3 adrenoreceptor agonist, was reported to be efficacious, with a relatively safe drug profile, in elderly patients with OAB [44]. Desmopressin can also be used, but caution is needed in patients at risk of hyponatremia [44]. For those with nocturnal urgent urinary incontinence, which stems from the imbalance between bladder capacity and nocturnal urinary production, fesosterodine, an antimuscarinic drug, has been recommended for the alleviation of symptoms [45]. A dose of 4 mg of fesosterodine was effective in increasing sleep continuity when compared with that of controls [46]. Treatment of urgent urinary incontinence is critically important in OAB patients. There has been a report where pharmacological intervention in urgent urinary incontinence patients substantially improved sleep quality, duration, and efficiency [47]. Solifenacin, another competitive antimuscarinic drug, was effective in relieving sleep disturbance, not by decreasing nocturia episodes but by reducing urgency [48]. An oxybutynin patch, with its once-daily antimuscarinic property, was tested for efficacy in OAB patients [36]. When compared with controls, OAB patients with an oxybutynin patch showed fewer nocturia episodes and increased sleep continuity, which improved sleep-related quality of life [36].
The relationship between OSA, OAB, and nocturia has been discussed in some of the literature [49]. OSA is well known for its relationship to erectile dysfunction and nocturia. Severity of OSA was proportional to OAB and urinary incontinence occurrence [50]. A diluted nocturnal urine was predictive of OSA diagnosis, with 88% of sensitivity in patients with daytime OAB symptoms [51], which were more severe in the OSA group than in the control group [52]. Proposed mechanisms for the aforementioned relationship include the intermittent hypoxia induced by OSA; this oxidative stress changes the bladder structurally and functionally [53]. Indeed, bladder-wall thickness and OAB symptoms were closely related in OSA patients [52]. However, one study found no increased prevalence of OAB, nocturia, or urgent urinary incontinence symptoms in young male patients with OSA [54]. Further studies are warranted for the early diagnosis of OSA in OAB patients with nocturia and urgent urinary incontinence.
Diabetes Mellitus: Relationship with Nocturia, Sleep and OSA
Most literature on diabetes mellitus (DM) and nocturia is on type 2 DM, on which the following paragraphs will mostly focus. In a Japanese study that included 332 male type 2 DM patients, approximately 80% of the participants complained of nocturia [55]. In a recent study on type 2 DM patients, older age, increased hemoglobin A1c level, lower extremity edema, and peripheral neuropathy predicted nocturia [56]. Exact mechanisms for this finding have not been fully elucidated. In a relatively large study on 1301 type 2 DM patients, comorbid OAB was a predictor of nocturia with the highest odds, and type 2 DM patients with nocturia had a higher death rate, even allowing for age and DM duration [57].
Those with higher nocturia frequency showed higher hemoglobin A1c levels, an indication of poorer glycemic control [56]. In a study on 275 type 2 DM patients, those with nocturia complained of poorer sleep quality [58]. Poor glycemic control in type 2 DM patients experiencing nocturia might be attributable to poor sleep quality induced by nocturia episodes. A previous study accentuated the importance of sleep deprivation in the induction of obesity and resultant harmful effects on glycemic control [59]. Moreover, one study suggested that delaying time to first voiding during the night can significantly reduce blood glucose in type 2 DM patients [60]. Furthermore, sleep fragmentation induced by nocturia can increase the next-day fatigue and resultant sedentary activities, which can worsen glycemic control [61].
Meanwhile, as mentioned above, nocturia is an important clinical marker that strongly represents the diagnosis of OSA; hence nocturia in type 2 DM patients should not be dismissed. Indeed, a bidirectional relationship between OSA and type 2 DM has been replicated in previous studies. OSA can increase the incidence of type 2 DM with its worsening of glucose metabolism, and type 2 DM can increase the incidence of OSA because of diabetic neuropathy, which can perniciously affect respiratory control and upper airway reflexes [62,63]. Although some studies recommend CPAP for type 2 DM with OSA patients [64, 65], recent systematic reviews and meta-analyses present rather a disheartening result on the efficacy of CPAP on glycemic control of type 2 DM patients [66]. Still, considering the harmful effects of OSA on the clinical course of type 2 DM, it is essential to further evaluate for OSA diagnosis in those presenting with nocturia.
Depression: Relationship to Nocturia, Sleep, and OSA
Previous literature reported a close link between depression and nocturia. Major depression increased the odds of nocturia 6-fold, with proposed mechanisms involving disturbed antidiuretic hormone secretion and changes of CNS monoamine concentrations that can induce OAB, decrease reflex bladder contractions, and raise the bladder volume threshold [67]. Nocturia was not associated with the severity of depression, but there was a clear correlation between sleep quality and nocturia in patients with depression [68]. A bidirectional relationship also exists between depression and nocturia, with a report where LUTS including nocturia, when continued long term, can cause depression and anxiety [69].
Patients with depression on antidepressant medication should be asked about symptoms of nocturia. Even after controlling for depression, treatment with a selective serotonin reuptake inhibitor (SSRI) significantly increased the odds of nocturia [70]. Different classes of antidepressants can result in disparate prevalence of nocturia. One study reported a divergent result of nocturnal urinary frequency between those on SSRIs and those on noradrenaline reuptake inhibitors (NRI), with patients on SSRI reporting higher nocturnal urinary frequency than did those on NRI [71]. Moreover, compared with duloxetine, frequency increased 3-fold in the sertraline group [71]. These results arise from different drugs and side-effect profiles, but because nocturia increases depression and affects sleep quality in depressed patients, it is advisable to keep close surveillance of symptoms of nocturia in patients with depression.
Patients with both depressive symptoms and nocturia should always be asked for their sleep history and signs of OSA, since symptoms of OSA can mimic those of depression. About a quarter of male OSA patients reported low libido and had more nocturia events [72]. In a study where excessive daytime sleepiness exceeded the Epworth Sleepiness Scale, excessive daytime sleepiness was significantly associated with OSA severity and depression [73]. In a patient group with atypical depression, hypersomnia is often a presenting symptom. It is necessary to differentiate hypersomnia from excessive daytime sleepiness and to expand the assessment to include sleep-history taking and OSA diagnosis.
CONCLUSION
When a patient presents with symptoms of nocturia and sleep disturbance, a clinician should search for common comorbidities that can induce nocturia. Moreover, it is important to understand the bidirectionality of those common comorbidities and nocturia. Since severity of nocturia is closely related to sleep disturbance, clinicians should look for appropriate treatments that consider the common comorbidities addressed in this review, not focusing only on the sleep problem itself. Furthermore, nocturia as a core symptom of OSA should not be dismissed in clinical settings, since many comorbidities of nocturia have a close association with OSA.